Insulin signaling and the regulation of glucose transport
- PMID: 16307172
- PMCID: PMC1431367
- DOI: 10.2119/2005-00029.Saltiel
Insulin signaling and the regulation of glucose transport
Abstract
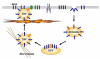
Gaps remain in our understanding of the precise molecular mechanisms by which insulin regulates glucose uptake in fat and muscle cells. Recent evidence suggests that insulin action involves multiple pathways, each compartmentalized in discrete domains. Upon activation, the receptor catalyzes the tyrosine phosphorylation of a number of substrates. One family of these, the insulin receptor substrate (IRS) proteins, initiates activation of the phosphatidylinositol 3-kinase pathway, resulting in stimulation of protein kinases such as Akt and atypical protein kinase C. The receptor also phosphorylates the adapter protein APS, resulting in the activation of the G protein TC10, which resides in lipid rafts. TC10 can influence a number of cellular processes, including changes in the actin cytoskeleton, recruitment of effectors such as the adapter protein CIP4, and assembly of the exocyst complex. These pathways converge to control the recycling of the facilitative glucose transporter Glut4.
Figures
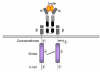

References
-
- Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. - PubMed
-
- Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–78. - PubMed
-
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. - PubMed
-
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–77. - PubMed
-
- Kandror KV, Pilch PF. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol. 1996;271:E1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
