Synchronized network activity in developing rat hippocampus involves regional hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function
- PMID: 16307610
- PMCID: PMC2927207
- DOI: 10.1111/j.1460-9568.2005.04407.x
Synchronized network activity in developing rat hippocampus involves regional hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function
Abstract
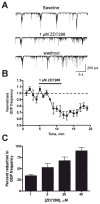
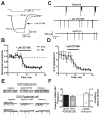
The principal form of synchronized network activity in neonatal hippocampus consists of low frequency 'giant depolarizing potentials' (GDPs). Whereas contribution of both GABA and glutamate to their generation has been demonstrated, full understanding of the mechanisms underlying these synchronized activity bursts remains incomplete. A contribution of the h-current, conducted by HCN channels, to GDPs has been a topic of substantial interest. Here we focus on HCN1, the prevalent HCN channel isoform in neonatal hippocampus, and demonstrate an HCN1 spatiotemporal expression pattern in both CA3 principal cells and interneurons that correlates with the developmental profile of GDPs. Abrogation of HCN physiological function in CA3, via the selective I(h)-blocker ZD7288, disrupts GDP generation. Furthermore, ZD7288 specifically abolishes spontaneous bursting of the CA3 pyramidal cells at frequencies typical of GDPs without major influence on interneuronal firing. These findings support a pivotal role for HCN channels expressed by CA3 neurons, and particularly CA3 pyramidal cells, in GDP-related network synchronization.
Figures

Similar articles
-
Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus.Cereb Cortex. 2007 Mar;17(3):702-12. doi: 10.1093/cercor/bhk021. Epub 2006 Apr 28. Cereb Cortex. 2007. PMID: 16648453 Free PMC article.
-
Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus.Neuroscience. 2001;106(4):689-98. doi: 10.1016/s0306-4522(01)00314-1. Neuroscience. 2001. PMID: 11682156 Free PMC article.
-
Single channel properties of hyperpolarization-activated cation currents in acutely dissociated rat hippocampal neurones.J Physiol. 2005 Oct 15;568(Pt 2):371-80. doi: 10.1113/jphysiol.2005.093161. Epub 2005 Aug 25. J Physiol. 2005. PMID: 16123099 Free PMC article.
-
GABAergic control of CA3-driven network events in the developing hippocampus.Results Probl Cell Differ. 2008;44:99-121. doi: 10.1007/400_2007_033. Results Probl Cell Differ. 2008. PMID: 17622497 Review.
-
Cardiac HCN channels: structure, function, and modulation.Trends Cardiovasc Med. 2002 Jul;12(5):206-12. doi: 10.1016/s1050-1738(02)00162-7. Trends Cardiovasc Med. 2002. PMID: 12161074 Review.
Cited by
-
The depolarizing action of GABA controls early network activity in the developing hippocampus.Mol Neurobiol. 2011 Apr;43(2):97-106. doi: 10.1007/s12035-010-8147-z. Epub 2010 Nov 3. Mol Neurobiol. 2011. PMID: 21042953 Review.
-
Differential expression of hyperpolarization-activated cyclic nucleotide-gated channel subunits during hippocampal development in the mouse.Mol Brain. 2015 Feb 27;8:13. doi: 10.1186/s13041-015-0103-4. Mol Brain. 2015. PMID: 25761792 Free PMC article.
-
A novel, noninvasive, predictive epilepsy biomarker with clinical potential.J Neurosci. 2014 Jun 25;34(26):8672-84. doi: 10.1523/JNEUROSCI.4806-13.2014. J Neurosci. 2014. PMID: 24966369 Free PMC article.
-
Development of theta rhythmicity in entorhinal stellate cells of the juvenile rat.J Neurophysiol. 2008 Dec;100(6):3144-57. doi: 10.1152/jn.90424.2008. Epub 2008 Oct 1. J Neurophysiol. 2008. PMID: 18829850 Free PMC article.
-
Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus.Eur J Neurosci. 2006 Jul;24(1):94-104. doi: 10.1111/j.1460-9568.2006.04880.x. Eur J Neurosci. 2006. PMID: 16882011 Free PMC article.
References
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
-
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal hippocampal slices. J Neurophysiol. 1999;81:2095–2102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

