The impact of clinical trials on the use of hormone replacement therapy. A population-based study
- PMID: 16307628
- PMCID: PMC1490267
- DOI: 10.1111/j.1525-1497.2005.0221.x
The impact of clinical trials on the use of hormone replacement therapy. A population-based study
Abstract
Background: The last 5 years of trial data demonstrate the ineffectiveness of hormone replacement therapy (HRT). The impact of these trials on age-specific HRT use, HRT discontinuation, and regional HRT variation has not been evaluated extensively.
Objective: To characterize the relation between HRT trial dissemination and age-specific HRT use, HRT discontinuation, and regional HRT variation before and after the trials' publication.
Design: Using the Medco Health database, we analyzed HRT prescription filling, discontinuation, and regional variation among women > or =55 years from May 1998 to May 2003.
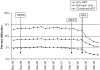
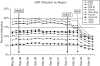
Measurements and main results: Approximately 340,000 women were eligible for Medco benefits each month. Within 3 months of the Women's Health Initiative (WHI), HRT prescriptions declined from 12.5% to 9.4%, P< or =.0001. When stratified by age, a statistically significant decline in HRT post-WHI occurred in all age groups, with the biggest decline among women > or =55 to 64 (18% to 11%, P< or =.0001). Among HRT users, we found statistically significant increases in discontinuation in 2002 (67%) compared with 2001 (53%, P<.0001). Prior to the WHI there was substantial regional variation in HRT use, with the West South Central and mid-Atlantic having the highest and lowest proportions, respectively (19% vs 6%, P< or =.0001). Despite a relative decline in HRT use of 25% to 42% across all regions, substantial geographic variation remained.
Conclusions: Hormone replacement therapy use decreased significantly immediately post-WHI, suggesting that trial results can have a rapid effect on practice. Marked regional variation in HRT use persisted after the WHI, suggesting that local practice patterns exert a strong effect on clinical behavior even after new evidence is available.
Figures


References
-
- Lenfant C. Clinical research to clinical practice—lost in translation? N Engl J Med. 2003;349:868–74. - PubMed
-
- Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–75. - PubMed
-
- Col NF, Eckman MH, Karas RH, et al. Patient-specific decisions about hormone replacement therapy in postmenopausal women. JAMA. 1997;277:1140–7. - PubMed
-
- Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the Nurses' Health Study. N Engl J Med. 1991;325:756–62. - PubMed
-
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitte D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
