A role for 5,6-epoxyeicosatrienoic acid in calcium entry by de novo conformational coupling in human platelets
- PMID: 16308346
- PMCID: PMC1464301
- DOI: 10.1113/jphysiol.2005.100800
A role for 5,6-epoxyeicosatrienoic acid in calcium entry by de novo conformational coupling in human platelets
Abstract
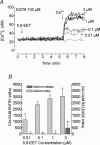
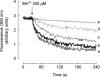
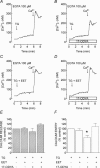
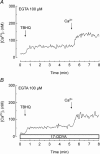
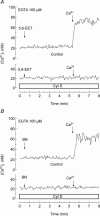
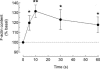
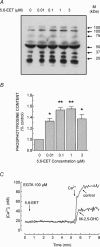
A major pathway for Ca(2+) entry in non-excitable cells is activated following depletion of intracellular Ca(2+) stores. A de novo conformational coupling between elements in the plasma membrane (PM) and Ca(2+) stores has been proposed as the most likely mechanism to activate this capacitative Ca(2+) entry (CCE) in several cell types, including platelets. Here we report that a cytochrome P450 metabolite, 5,6-EET, might be a component of the de novo conformational coupling in human platelets. In these cells, 5,6-EET induces divalent cation entry without having any detectable effect on Ca(2+) store depletion. 5,6-EET-induced Ca(2+) entry was sensitive to the CCE blockers 2-APB, lanthanum, SKF-96365 and nickel and impaired by incubation with anti-hTRPC1 antibody. Ca(2+) entry stimulated by low concentrations of thapsigargin, which selectively depletes the dense tubular system and induces EET production, was impaired by the cytochrome P450 inhibitor 17-ODYA, which has no effect on CCE mediated by depletion of the acidic stores using 2,5-di-(tert-butyl)-1,4-hydroquinone. We have found that 5,6-EET-induced Ca(2+) entry requires basal levels of H(2)O(2), which might maintain a redox state favourable for this event. Finally, our results indicate that 5,6-EET induces the activation of tyrosine kinase proteins and the reorganization of the actin cytoskeleton, which might provide a support for the transport of portions of the Ca(2+) store towards the PM to facilitate de novo coupling between IP(3)R type II and hTRPC1 detected by coimmunoprecipitation. We propose that the involvement of 5,6-EET in TG-induced coupling between IP(3)R type II and hTRPC1 and subsequently CCE is compatible with the de novo conformational coupling in human platelets.
Figures



References
-
- Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:445–451. - PubMed
-
- Bergdhal A, Gómez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. - PubMed
-
- Bird GS, Putney JW. Inhibition of thapsigargin-induced calcium entry by microinjected guanine nucleotide analogues. Evidence for the involvement of a small G-protein in capacitative calcium entry. J Biol Chem. 1993;268:21486–21488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

