Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia
- PMID: 16308564
- PMCID: PMC1356326
- DOI: 10.1038/sj.emboj.7600885
Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia
Abstract
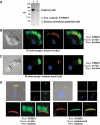
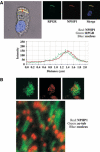
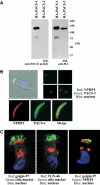
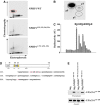
Mutations in proteins localized to cilia and basal bodies have been implicated in a growing number of human diseases. Access of these proteins to the ciliary compartment requires targeting to the base of the cilia. However, the mechanisms involved in transport of cilia proteins to this transitional zone are elusive. Here we show that nephrocystin, a ciliary protein mutated in the most prevalent form of cystic kidney disease in childhood, is expressed in respiratory epithelial cells and accumulates at the base of cilia, overlapping with markers of the basal body area and the transition zone. Nephrocystin interacts with the phosphofurin acidic cluster sorting protein (PACS)-1. Casein kinase 2 (CK2)-mediated phosphorylation of three critical serine residues within a cluster of acidic amino acids in nephrocystin mediates PACS-1 binding, and is essential for colocalization of nephrocystin with PACS-1 at the base of cilia. Inhibition of CK2 activity abrogates this interaction and results in the loss of correct nephrocystin targeting. These data suggest that CK2-dependent transport processes represent a novel pathway of targeting proteins to the cilia.
Figures



References
-
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425: 628–633 - PubMed
-
- Benzing T, Brandes R, Sellin L, Schermer B, Lecker S, Walz G, Kim E (1999) Upregulation of RGS7 may contribute to tumor necrosis factor-induced changes in central nervous function. Nat Med 5: 913–918 - PubMed
-
- Benzing T, Yaffe MB, Arnould T, Sellin L, Schermer B, Schilling B, Schreiber R, Kunzelmann K, Leparc GG, Kim E, Walz G (2000) 14-3-3 interacts with regulator of G protein signaling proteins and modulates their activity. J Biol Chem 275: 28167–28172 - PubMed
-
- Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G (2002) HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111: 853–866 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

