TLR agonists regulate PDGF-B production and cell proliferation through TGF-beta/type I IFN crosstalk
- PMID: 16308570
- PMCID: PMC1356307
- DOI: 10.1038/sj.emboj.7600867
TLR agonists regulate PDGF-B production and cell proliferation through TGF-beta/type I IFN crosstalk
Abstract
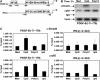
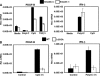
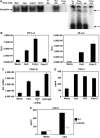
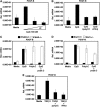
Transforming growth factor-beta (TGF-beta) and type I interferon (IFN) autocrine/paracrine loops are recognized as key mediators of signaling cascades that control a variety of cellular functions. Here, we describe a novel mechanism by which Toll-like receptor (TLR) agonists utilize these two autocrine/paracrine loops to differentially regulate the induction of PDGF-B, a growth factor implicated in a number of diseases ranging from tumor metastasis to glomerulonephritis. We demonstrate that CpG-specific induction of PDGF-B requires activation of Smads through TGFbeta1 autocrine/paracrine signaling. In contrast, polyinosinic:polycytidylic acid strongly represses CpG's as well as its own intrinsic ability to induce PDGF-B mRNA through type I IFN-mediated induction of Smad7, a negative regulator of Smad3/4. Furthermore, we have shown that this crosstalk mechanism translates into similar regulation of mesangial cell proliferation. Thus, our results demonstrate the importance of crosstalk between TGF-beta and type I IFNs in determining the specificity of TLR-mediated gene induction.
Figures



Similar articles
-
Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway.Science. 2003 Aug 1;301(5633):640-3. doi: 10.1126/science.1087262. Epub 2003 Jul 10. Science. 2003. PMID: 12855817
-
Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway.Biochem Biophys Res Commun. 2006 Feb 17;340(3):742-50. doi: 10.1016/j.bbrc.2005.12.069. Epub 2005 Dec 20. Biochem Biophys Res Commun. 2006. PMID: 16380081
-
Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro.Kidney Int. 2012 Feb;81(3):266-79. doi: 10.1038/ki.2011.327. Epub 2011 Nov 2. Kidney Int. 2012. PMID: 22048127
-
Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction.J Exp Med. 2005 Mar 21;201(6):915-23. doi: 10.1084/jem.20042372. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767370 Free PMC article.
-
SMAD7: at the interface of TGF-beta and proinflammatory signaling.Perit Dial Int. 2007 Sep-Oct;27(5):523-5. Perit Dial Int. 2007. PMID: 17704440 Review. No abstract available.
Cited by
-
TLR3 deficiency protects against collagen degradation and medial destruction in murine atherosclerotic plaques.Atherosclerosis. 2013 Jul;229(1):52-61. doi: 10.1016/j.atherosclerosis.2013.03.035. Epub 2013 Apr 9. Atherosclerosis. 2013. PMID: 23676255 Free PMC article.
-
TNF-α expression in Schwann cells is induced by LPS and NF-κB-dependent pathways.Neurochem Res. 2012 Apr;37(4):722-31. doi: 10.1007/s11064-011-0664-2. Epub 2012 Jan 5. Neurochem Res. 2012. PMID: 22219126
-
Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity.Sci Rep. 2020 Jan 20;10(1):670. doi: 10.1038/s41598-019-57368-w. Sci Rep. 2020. PMID: 31959796 Free PMC article.
-
Nucleotide-binding oligomerization domain protein 2 deficiency enhances neointimal formation in response to vascular injury.Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2441-7. doi: 10.1161/ATVBAHA.111.235135. Arterioscler Thromb Vasc Biol. 2011. PMID: 21903945 Free PMC article.
-
IFN-I Mediates Lupus Nephritis From the Beginning to Renal Fibrosis.Front Immunol. 2021 Apr 20;12:676082. doi: 10.3389/fimmu.2021.676082. eCollection 2021. Front Immunol. 2021. PMID: 33959133 Free PMC article. Review.
References
-
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H (2002) Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol 32: 1958–1968 - PubMed
-
- Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680 - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 - PubMed
-
- Blom IE, van Dijk AJ, Wieten L, Duran K, Ito Y, Kleij L, deNichilo M, Rabelink TJ, Weening JJ, Aten J, Goldschmeding R (2001) In vitro evidence for differential involvement of CTGF, TGFbeta, and PDGF-BB in mesangial response to injury. Nephrol Dial Transplant 16: 1139–1148 - PubMed
-
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y (2004) PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6: 333–345 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

