Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomospis asparagi
- PMID: 16309305
- PMCID: PMC3972004
- DOI: 10.1021/np050293f
Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomospis asparagi
Abstract
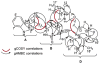
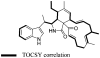
The isolation and structure elucidation of three new secondary metabolites, chaetoglobosin-510 (1), -540 (2), and -542 (3), are described. These compounds were produced by cultures of the marine-derived fungus Phomopsis asparagi, challenged with the known F-actin inhibitor jasplakinolide. Chaetoglobosin-542 (3) displayed antimicrofilament activity and was cytotoxic toward murine colon and leukemia cancer cell lines.
Figures
References
-
- Firn RD, Jones CG. Nat Prod Rep. 2003;20:382–391. - PubMed
-
-
This approach has been named OSMAC: Bode HB, Bethe B, Höfs R, Zeeck A. ChemBioChem. 2002;3:691–627.
-
-
- Preisg CL, Laakso JA, Mocek UM, Wang PT, Baez J, Byng G. J Nat Prod. 2003;66:350–356. - PubMed
- Okuno Y, Miyazawa M. J Nat Prod. 2004;67:1876–1878. - PubMed
- Zhang J, Cheng ZH, Yu BY, Cordell GA, Qiu SX. Tetrahedron Lett. 2005;46:2337–2340.
- Tian Y, Guo H, Han J, Guo D. J Nat Prod. 2005;68:678–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources