Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities
- PMID: 16311519
- PMCID: PMC1369227
- DOI: 10.1038/sj.embor.7400573
Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities
Abstract
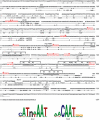
Myelination in Schwann cells is governed by several transcription factors, including the POU proteins Oct6 and Brn2, the high mobility group protein Sox10 and the zinc-finger protein Krox20. How the function of these factors is integrated in the control of myelination has not been established. Previously, we identified an enhancer element controlling Krox20 expression throughout myelination in Schwann cells. In this paper, cell culture experiments were combined with transgenesis to identify transcription factors acting directly upstream of Krox20. The results show that during the promyelin-myelin transition, Krox20 expression is directly activated by Oct6 and Brn2 acting on this enhancer. In addition, the enhancer-dependent synergism between these POU proteins and Sox10 suggests that Krox20 expression requires this combination of factors. These results resolve previous controversy concerning the mechanism of action of Oct6 and Brn2 during myelination and provide an explanation for myelin deficiencies in Waardenberg-Hirschsprung disease patients whereby Sox10 mutations could lead to a loss of Krox20 expression.
Figures

References
-
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M (2002) Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development 129: 155–166 - PubMed
-
- Ghislain J, Desmarquet-Trin-Dinh C, Gilardi-Hebenstreit P, Charnay P, Frain M (2003) Neural crest patterning: autoregulatory and crest-specific elements co-operate for Krox20 transcriptional control. Development 130: 941–953 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

