Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice
- PMID: 16311599
- PMCID: PMC3057888
- DOI: 10.1038/ni1289
Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice
Abstract
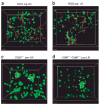
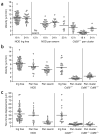
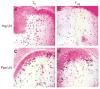
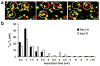
The in vivo mechanism of regulatory T cell (T(reg) cell) function in controlling autoimmunity remains controversial. Here we have used two-photon laser-scanning microscopy to analyze lymph node priming of diabetogenic T cells and to delineate the mechanisms of T(reg) cell control of autoimmunity in vivo. Islet antigen-specific CD4(+)CD25(-) T helper cells (T(H) cells) and T(reg) cells swarmed and arrested in the presence of autoantigens. These T(H) cell activities were progressively inhibited in the presence of increasing numbers of T(reg) cells. There were no detectable stable associations between T(reg) and T(H) cells during active suppression. In contrast, T(reg) cells directly interacted with dendritic cells bearing islet antigen. Such persistent T(reg) cell-dendritic cell contacts preceded the inhibition of T(H) cell activation by dendritic cells, supporting the idea that dendritic cells are central to T(reg) cell function in vivo.
Conflict of interest statement
Figures




Comment in
-
Lymph node choreography: Treg cells join the dance.Nat Immunol. 2006 Jan;7(1):11-3. doi: 10.1038/ni0106-11. Nat Immunol. 2006. PMID: 16357850 No abstract available.
References
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells–they're back and critical for regulation of autoimmunity! Immunol Rev. 2001;182:149–163. - PubMed
-
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. - PubMed
-
- Singh B, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. - PubMed
-
- Curotto de Lafaille MA, Lafaille JJ. CD4+ regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–778. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

