Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor
- PMID: 16311903
- PMCID: PMC1633720
- DOI: 10.1007/s11010-005-8050-5
Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor
Abstract
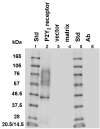
Purification of HA-tagged P2Y2 receptors from transfected human 1321N1 astrocytoma cells yielded a protein with a molecular size determined by SDS-PAGE to be in the range of 57-76 kDa, which is typical of membrane glycoproteins with heterogeneous complex glycosylation. The protein phosphatase inhibitor, okadaic acid, attenuated the recovery of receptor activity from the agonist-induced desensitized state, suggesting a role for P2Y2 receptor phosphorylation in desensitization. Isolation of HA-tagged P2Y2 nucleotide receptors from metabolically [32P]-labelled cells indicated a (3.8 +/- 0.2)-fold increase in the [32P]-content of the receptor after 15 min of treatment with 100 microM UTP, as compared to immunoprecipitated receptors from untreated control cells. Receptor sequestration studies indicated that approximately 40% of the surface receptors were internalized after a 15-min stimulation with 100 microM UTP. Point mutation of three potential GRK and PKC phosphorylation sites in the third intracellular loop and C-terminal tail of the P2Y2 receptor (namely, S243A, T344A, and S356A) extinguished agonist-induced receptor phosphorylation, caused a marked reduction in the efficacy of UTP to desensitize P2Y2 receptor signalling to intracellular calcium mobilization, and impaired agonist-induced receptor internalization. Activation of PKC isoforms with phorbol 12-myristate 13-acetate that caused heterologous receptor desensitization did not increase the level of P2Y2 receptor phosphorylation. Our results indicate a role for receptor phosphorylation by phorbol-insensitive protein kinases in agonist-induced desensitization of the P2Y2 nucleotide receptor.
Figures

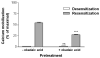






Similar articles
-
Mechanisms of agonist-dependent and -independent desensitization of a recombinant P2Y2 nucleotide receptor.Mol Cell Biochem. 2000 Feb;205(1-2):115-23. doi: 10.1023/a:1007018001735. Mol Cell Biochem. 2000. PMID: 10821429
-
P2Y(2) nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases.J Cell Physiol. 2001 May;187(2):196-208. doi: 10.1002/jcp.1063. J Cell Physiol. 2001. PMID: 11267999
-
Differential agonist-induced desensitization of P2Y2 nucleotide receptors by ATP and UTP.Mol Cell Biochem. 2000 Mar;206(1-2):75-89. doi: 10.1023/a:1007091127392. Mol Cell Biochem. 2000. PMID: 10839197
-
Mechanisms for inhibition of P2 receptors signaling in neural cells.Mol Neurobiol. 2005;31(1-3):65-79. doi: 10.1385/MN:31:1-3:065. Mol Neurobiol. 2005. PMID: 15953812 Review.
-
P2Y2 Receptor Functions in Cancer: A Perspective in the Context of Colorectal Cancer.Adv Exp Med Biol. 2017;1051:91-106. doi: 10.1007/5584_2017_90. Adv Exp Med Biol. 2017. PMID: 28815512 Review.
Cited by
-
Streptococcus pneumoniae inhibits purinergic signaling and promotes purinergic receptor P2Y2 internalization in alveolar epithelial cells.J Biol Chem. 2019 Aug 23;294(34):12795-12806. doi: 10.1074/jbc.RA118.007236. Epub 2019 Jul 9. J Biol Chem. 2019. PMID: 31289122 Free PMC article.
-
P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):722-38. doi: 10.2174/187152712803581047. CNS Neurol Disord Drug Targets. 2012. PMID: 22963441 Free PMC article. Review.
-
P2 receptors: intracellular signaling.Pflugers Arch. 2006 Aug;452(5):552-62. doi: 10.1007/s00424-006-0069-2. Epub 2006 Apr 4. Pflugers Arch. 2006. PMID: 16586093 Review.
-
Physiology and pathophysiology of human airway mucus.Physiol Rev. 2022 Oct 1;102(4):1757-1836. doi: 10.1152/physrev.00004.2021. Epub 2022 Jan 10. Physiol Rev. 2022. PMID: 35001665 Free PMC article. Review.
-
The P2Y2 Receptor C-Terminal Tail Modulates but Is Dispensable for β-Arrestin Recruitment.Int J Mol Sci. 2022 Mar 22;23(7):3460. doi: 10.3390/ijms23073460. Int J Mol Sci. 2022. PMID: 35408820 Free PMC article.
References
-
- Kannan S. Neutrophil chemotaxis: potential role of chemokine receptors in extracellular nucleotide induced Mac-1 expression. Med Hypotheses. 2003;61:577–579. - PubMed
-
- Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol (Berl) 1994;87:8–13. - PubMed
-
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

