Relations and interactions between cranial mesoderm and neural crest populations
- PMID: 16313393
- PMCID: PMC1571569
- DOI: 10.1111/j.1469-7580.2005.00473.x
Relations and interactions between cranial mesoderm and neural crest populations
Abstract
The embryonic head is populated by two robust mesenchymal populations, paraxial mesoderm and neural crest cells. Although the developmental histories of each are distinct and separate, they quickly establish intimate relations that are variably important for the histogenesis and morphogenesis of musculoskeletal components of the calvaria, midface and branchial regions. This review will focus first on the genesis and organization within nascent mesodermal and crest populations, emphasizing interactions that probably initiate or augment the establishment of lineages within each. The principal goal is an analysis of the interactions between crest and mesoderm populations, from their first contacts through their concerted movements into peripheral domains, particularly the branchial arches, and continuing to stages at which both the differentiation and the integrated three-dimensional assembly of vascular, connective and muscular tissues is evident. Current views on unresolved or contentious issues, including the relevance of head somitomeres, the processes by which crest cells change locations and constancy of cell-cell relations at the crest-mesoderm interface, are addressed.
Figures


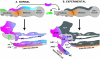
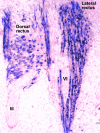
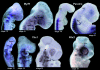
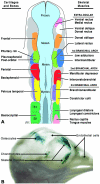
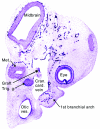
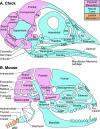
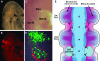

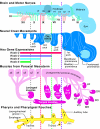
References
-
- Adelmann H. The development of the eye muscles of the chick. J Morph Physol. 1927;44:29–87.
-
- Anderson CB, Meier S. The influence of the metameric pattern in the mesoderm on migration of cranial neural crest cells in the chick embryo. Dev Biol. 1981;85:385–402. - PubMed
-
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith JB. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. - PubMed
-
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. - PubMed
-
- Baker RK, Antin PB. Ephs and ephrins during early stages of chick embryogenesis. Dev Dyn. 2003;228:128–142. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

