Characterization of point mutations in the cdtA gene of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans
- PMID: 16313618
- PMCID: PMC1435350
- DOI: 10.1111/j.1365-2958.2005.04905.x
Characterization of point mutations in the cdtA gene of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans
Abstract
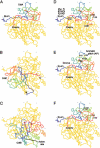
The Cdt is a family of gram-negative bacterial toxins that typically arrest eukaryotic cells in the G0/G1 or G2/M phase of the cell cycle. The toxin is a heterotrimer composed of the cdtA, cdtB and cdtC gene products. Although it has been shown that the CdtA protein subunit binds to cells in culture and in an enzyme-linked immunosorbent assay (CELISA) the precise mechanisms by which CdtA interacts with CdtB and CdtC has not yet been clarified. In this study we employed a random mutagenesis strategy to construct a library of point mutations in cdtA to assess the contribution of individual amino acids to binding activity and to the ability of the subunit to form biologically active holotoxin. Single unique amino acid substitutions in seven CdtA mutants resulted in reduced binding of the purified recombinant protein to Chinese hamster ovary cells and loss of binding to the fucose-containing glycoprotein, thyroglobulin. These mutations clustered at the 5'- and 3'-ends of the cdtA gene resulting in amino acid substitutions that resided outside of the aromatic patch region and a conserved region in CdtA homologues. Three of the amino acid substitutions, at positions S165N (mutA81), T41A (mutA121) and C178W (mutA221) resulted in gene products that formed holotoxin complexes that exhibited a 60% reduction (mutA81) or loss (mutA121, mutA221) of proliferation inhibition. A similar pattern was observed when these mutant holotoxins were tested for their ability to induce cell cycle arrest and to convert supercoiled DNA to relaxed and linear forms in vitro. The mutations in mutA81 and mutA221 disrupted holotoxin formation. The positions of the amino acid substitutions were mapped in the Haemophilus ducreyi Cdt crystal structure providing some insight into structure and function.
Figures








References
-
- Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect Immun. 2001;69:5925–5930. - PMC - PubMed
-
- Akifusa S, Heywood W, Nair SP, Stenbeck G, Henderson B. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology. 2005;151:1395–1402. - PubMed
-
- Cortes-Bratti X, Frisan T, Thelestam M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon. 2001a;39:1729–1736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

