Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core
- PMID: 16314458
- PMCID: PMC1370875
- DOI: 10.1261/rna.2155905
Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core
Abstract
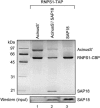
The multiprotein exon junction complex (EJC) is deposited on mRNAs upstream of exon-exon junctions as a consequence of pre-mRNA splicing. In mammalian cells, this complex serves as a key modulator of spliced mRNA metabolism. To date, neither the complete composition nor the exact assembly pathway of the EJC has been entirely elucidated. Using in vitro splicing and a two-step chromatography procedure, we have purified the EJC and analyzed its components by mass spectrometry. In addition to finding most of the known EJC factors, we identified two novel EJC components, Acinus and SAP18. Heterokaryon analysis revealed that SAP18 is a shuttling protein whereas Acinus is restricted to the nucleus. In MS2 tethering assays Acinus stimulated gene expression at the RNA level, while MLN51, another EJC factor, stimulated mRNA translational efficiency. Using tandem affinity purification (TAP) of proteins overexpressed in HeLa cells, we demonstrated that Acinus binds directly to another EJC component, RNPS1, while stable association of SAP18 to form the trimeric apoptosis and splicing associated protein (ASAP) complex requires both Acinus and RNPS1. Using the same methodology, we further identified what appears to be the minimal stable EJC core, a heterotetrameric complex consisting of eIF4AIII, Magoh, Y14, and MLN51.
Figures

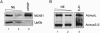
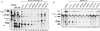




References
-
- Ballut, L., Marchadier, B., Baguet, A., Tomasetto, C., Seraphin, B., and Le Hir, H. 2005. The exon junction core complex is locked onto RNA by inhibition of EIF4AIII ATPase activity. Nat. Struct. Mol. Biol. 12: 861–869. - PubMed
-
- Conti, E. and Izaurralde, E. 2005. Nonsense-mediated mRNA decay: Molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 17: 316–325. - PubMed
-
- Degot, S., Le Hir, H., Alpy, F., Kedinger, V., Stoll, I., Wendling, C., Seraphin, B., Rio, M.C., and Tomasetto, C. 2004. Association of the breast cancer protein MLN51 with the exon junction complex via its speckle localizer and RNA binding module. J. Biol. Chem. 279: 33702–33715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
