Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo
- PMID: 16314471
- PMCID: PMC1613189
- DOI: 10.1016/S0002-9440(10)61242-4
Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo
Abstract
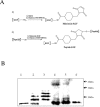
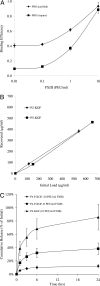
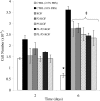
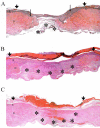
Exogenous keratinocyte growth factor (KGF) significantly enhances wound healing, but its use is hampered by a short biological half-life and lack of tissue selectivity. We used a biomimetic approach to achieve cell-controlled delivery of KGF by covalently attaching a fluorescent matrix-binding peptide that contained two domains: one recognized by factor XIII and the other by plasmin. Modified KGF was incorporated into the fibrin matrix at high concentration in a factor XIII-dependent manner. Cell-mediated activation of plasminogen to plasmin degraded the fibrin matrix and cleaved the peptides, releasing active KGF to the local microenvironment and enhancing epithelial cell proliferation and migration. To demonstrate in vivo effectiveness, we used a hybrid model of wound healing that involved transplanting human bioengineered skin onto athymic mice. At 6 weeks after grafting, the transplanted tissues underwent full thickness wounding and treatment with fibrin gels containing bound KGF. In contrast to topical KGF, fibrin-bound KGF persisted in the wounds for several days and was released gradually, resulting in significantly enhanced wound closure. A fibrinolytic inhibitor prevented this healing, indicating the requirement for cell-mediated fibrin degradation to release KGF. In conclusion, this biomimetic approach of localized, cell-controlled delivery of growth factors may accelerate healing of large full-thickness wounds and chronic wounds that are notoriously difficult to heal.
Figures




References
-
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. - PubMed
-
- Collen D. Ham-Wasserman lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology (Am Soc Hematol Educ Program) 2001:1–9. - PubMed
-
- Aaronson SA, Bottaro DP, Miki T, Ron D, Finch PW, Fleming TP, Ahn J, Taylor WG, Rubin JS. Keratinocyte growth factor. A fibroblast growth factor family member with unusual target cell specificity. Ann NY Acad Sci. 1991;638:62–77. - PubMed
-
- Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–165. - PubMed
-
- Rubin JS, Bottaro DP, Chedid M, Miki T, Ron D, Cheon G, Taylor WG, Fortney E, Sakata H, Finch PW, LaRochelle WJ. Keratinocyte growth factor. Cell Biol Int. 1995;19:399–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

