Specific sequence features, recognized by the SMN complex, identify snRNAs and determine their fate as snRNPs
- PMID: 16314521
- PMCID: PMC1316962
- DOI: 10.1128/MCB.25.24.10989-11004.2005
Specific sequence features, recognized by the SMN complex, identify snRNAs and determine their fate as snRNPs
Abstract
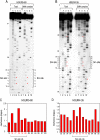
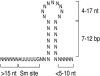
The survival of motor neurons (SMN) complex is essential for the biogenesis of spliceosomal small nuclear ribonucleoproteins (snRNPs) as it binds to and delivers Sm proteins for assembly of Sm cores on the abundant small nuclear RNAs (snRNAs). Using the conserved snRNAs encoded by the lymphotropic Herpesvirus saimiri (HVS), we determined the specific sequence and structural features of RNAs for binding to the SMN complex and for Sm core assembly. We show that the minimal SMN complex-binding domain in snRNAs, except U1, is comprised of an Sm site (AUUUUUG) and an adjacent 3' stem-loop. The adenosine and the first and third uridines of the Sm site are particularly critical for binding of the SMN complex, which directly contacts the backbone phosphates of these uridines. The specific sequence of the adjacent stem (7 to 12 base pairs)-loop (4 to 17 nucleotides) is not important for SMN complex binding, but it must be located within a short distance of the 3' end of the RNA for an Sm core to assemble. Importantly, these defining characteristics are discerned by the SMN complex and not by the Sm proteins, which can bind to and assemble on an Sm site sequence alone. These findings demonstrate that the SMN complex is the identifier, as well as assembler, of the abundant class of snRNAs in cells because it is able to recognize an snRNP code that they contain.
Figures










Similar articles
-
The SMN complex: an assembly machine for RNPs.Cold Spring Harb Symp Quant Biol. 2006;71:313-20. doi: 10.1101/sqb.2006.71.001. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381311 Review.
-
Lymphotropic Herpesvirus saimiri uses the SMN complex to assemble Sm cores on its small RNAs.Mol Cell Biol. 2005 Jan;25(2):602-11. doi: 10.1128/MCB.25.2.602-611.2005. Mol Cell Biol. 2005. PMID: 15632062 Free PMC article.
-
The Gemin5 protein of the SMN complex identifies snRNAs.Mol Cell. 2006 Jul 21;23(2):273-9. doi: 10.1016/j.molcel.2006.05.036. Mol Cell. 2006. PMID: 16857593
-
Why do cells need an assembly machine for RNA-protein complexes?Trends Cell Biol. 2004 May;14(5):226-32. doi: 10.1016/j.tcb.2004.03.010. Trends Cell Biol. 2004. PMID: 15130578 Review.
-
snRNAs contain specific SMN-binding domains that are essential for snRNP assembly.Mol Cell Biol. 2004 Apr;24(7):2747-56. doi: 10.1128/MCB.24.7.2747-2756.2004. Mol Cell Biol. 2004. PMID: 15024064 Free PMC article.
Cited by
-
Identification of gemin5 as a novel 7-methylguanosine cap-binding protein.PLoS One. 2009 Sep 14;4(9):e7030. doi: 10.1371/journal.pone.0007030. PLoS One. 2009. PMID: 19750007 Free PMC article.
-
RNA-binding proteins in disease etiology: fragile X syndrome and spinal muscular atrophy.RNA. 2025 Feb 19;31(3):277-283. doi: 10.1261/rna.080353.124. RNA. 2025. PMID: 39694825 Free PMC article. Review.
-
A unique mechanism of snRNP core assembly.Nat Commun. 2025 Apr 2;16(1):3166. doi: 10.1038/s41467-025-58461-7. Nat Commun. 2025. PMID: 40175367 Free PMC article.
-
Formation of the 3' end of histone mRNA: getting closer to the end.Gene. 2007 Jul 15;396(2):373-90. doi: 10.1016/j.gene.2007.04.021. Epub 2007 May 4. Gene. 2007. PMID: 17531405 Free PMC article. Review.
-
Gemin5: A Multitasking RNA-Binding Protein Involved in Translation Control.Biomolecules. 2015 Apr 17;5(2):528-44. doi: 10.3390/biom5020528. Biomolecules. 2015. PMID: 25898402 Free PMC article. Review.
References
-
- Baccon, J., L. Pellizzoni, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J. Biol. Chem. 277:31957-31962. - PubMed
-
- Buhler, D., V. Raker, R. Luhrmann, and U. Fischer. 1999. Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet. 8:2351-2357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
