Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins
- PMID: 16314572
- PMCID: PMC1308899
- DOI: 10.1073/pnas.0506010102
Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins
Abstract
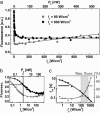
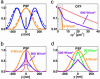
Fluorescence microscopy is indispensable in many areas of science, but until recently, diffraction has limited the resolution of its lens-based variant. The diffraction barrier has been broken by a saturated depletion of the marker's fluorescent state by stimulated emission, but this approach requires picosecond laser pulses of GW/cm2 intensity. Here, we demonstrate the surpassing of the diffraction barrier in fluorescence microscopy with illumination intensities that are eight orders of magnitude smaller. The subdiffraction resolution results from reversible photoswitching of a marker protein between a fluorescence-activated and a nonactivated state, whereby one of the transitions is accomplished by means of a spatial intensity distribution featuring a zero. After characterizing the switching kinetics of the used marker protein asFP595, we demonstrate the current capability of this RESOLFT (reversible saturable optical fluorescence transitions) type of concept to resolve 50-100 nm in the focal plane. The observed resolution is limited only by the photokinetics of the protein and the perfection of the zero. Our results underscore the potential to finally achieve molecular resolution in fluorescence microscopy by technical optimization.
Figures
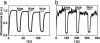

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

