Pathophysiology of hereditary hemochromatosis
- PMID: 16315135
- PMCID: PMC2587012
- DOI: 10.1055/s-2005-923313
Pathophysiology of hereditary hemochromatosis
Abstract
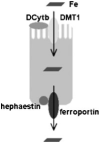
Hereditary hemochromatosis (HH) encompasses several inherited disorders of iron homeostasis characterized by increased gastrointestinal iron absorption and tissue iron deposition. The most common form of this disorder is HFE-related HH, nearly always caused by homozygosity for the C282Y mutation. A substantial proportion of C282Y homozygotes do not develop clinically significant iron overload, suggesting roles for environmental factors and modifier genes in determining the phenotype. Recent studies have demonstrated that the pathogenesis of nearly all forms of HH involves inappropriately decreased expression of the iron-regulatory hormone hepcidin. Hepcidin serves to decrease the export of iron from reticuloendothelial cells and absorptive enterocytes. Thus, HH patients demonstrate increased iron release from these cell types, elevated circulating iron, and iron deposition in vulnerable tissues. The mechanism by which HFE influences hepcidin expression is an area of current investigation and may offer insights into the phenotypic variability observed in persons with mutations in HFE.
Figures