The extracellular adherence protein (Eap) of Staphylococcus aureus inhibits wound healing by interfering with host defense and repair mechanisms
- PMID: 16317095
- PMCID: PMC1895382
- DOI: 10.1182/blood-2005-08-3140
The extracellular adherence protein (Eap) of Staphylococcus aureus inhibits wound healing by interfering with host defense and repair mechanisms
Abstract
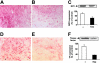
Staphylococcus aureus is a major human pathogen interfering with host-cell functions. Impaired wound healing is often observed in S aureus-infected wounds, yet, the underlying mechanisms are poorly defined. Here, we identify the extracellular adherence protein (Eap) of S aureus to be responsible for impaired wound healing. In a mouse wound-healing model wound closure was inhibited in the presence of wild-type S aureus and this effect was reversible when the wounds were incubated with an isogenic Eap-deficient strain. Isolated Eap also delayed wound closure. In the presence of Eap, recruitment of inflammatory cells to the wound site as well as neovascularization of the wound were prevented. In vitro, Eap significantly reduced intercellular adhesion molecule 1 (ICAM-1)-dependent leukocyte-endothelial interactions and diminished the consequent activation of the proinflammatory transcription factor nuclear factor kappaB (NFkappaB) in leukocytes associated with a decrease in expression of tissue factor. Moreover, Eap blocked alphav-integrin-mediated endothelial-cell migration and capillary tube formation, and neovascularization in matrigels in vivo. Collectively, the potent anti-inflammatory and antiangiogenic properties of Eap provide an underlying mechanism that may explain the impaired wound healing in S aureus-infected wounds. Eap may also serve as a lead compound for new anti-inflammatory and antiangiogenic therapies in several pathologies.
Figures
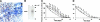
 ) of ICAM-1 (10 μg/mL), as indicated. The insert demonstrates a typical EMSA for NFκB DNA-binding activity (1 indicates control; 2, TNF-α; 3, TNF-α+Eap; 4, ICAM-1; 5, ICAM-1+TNFα; and 6, ICAM-1+TNFα+Eap). (D) The expression of tissue factor without or with TNFα or TNFα plus Eap in THP-1 cells is shown in the absence (□) or presence (
) of ICAM-1 (10 μg/mL), as indicated. The insert demonstrates a typical EMSA for NFκB DNA-binding activity (1 indicates control; 2, TNF-α; 3, TNF-α+Eap; 4, ICAM-1; 5, ICAM-1+TNFα; and 6, ICAM-1+TNFα+Eap). (D) The expression of tissue factor without or with TNFα or TNFα plus Eap in THP-1 cells is shown in the absence (□) or presence ( ) of ICAM-1 (10 μg/mL). Densitometric data are expressed relative to control (100% control is represented in the absence of stimuli or competitors), and are means ± SD of 2 separate experiments. *P < .05.
) of ICAM-1 (10 μg/mL). Densitometric data are expressed relative to control (100% control is represented in the absence of stimuli or competitors), and are means ± SD of 2 separate experiments. *P < .05.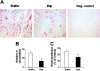
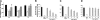
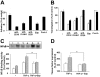
 ;20 μg/mL), or protein A (
;20 μg/mL), or protein A ( ;20 μg/mL). Cell migration is expressed relative to control, which is represented as cell migration in the absence of any stimulus or competitor. Data are means ± SD of 4 experiments performed in triplicate. *P < .05 compared with VEGF-stimulated migration in the absence of competitor. ns indicates nonsignificant. (B-D) Binding of VN to immobilized αvβ3-integrin (B), binding of FBG to immobilized αvβ3-integrin (C), and binding of FN to immobilized α5β1-integrin(D) was performed in the absence (–; ▪) or presence of blocking mAb against αvβ3-integrin, LM609 (for VN and FBG), the blocking mAb against β1-integrin, 6S6 (for FN) (20 μg/mL; □), or increasing concentrations of Eap (
;20 μg/mL). Cell migration is expressed relative to control, which is represented as cell migration in the absence of any stimulus or competitor. Data are means ± SD of 4 experiments performed in triplicate. *P < .05 compared with VEGF-stimulated migration in the absence of competitor. ns indicates nonsignificant. (B-D) Binding of VN to immobilized αvβ3-integrin (B), binding of FBG to immobilized αvβ3-integrin (C), and binding of FN to immobilized α5β1-integrin(D) was performed in the absence (–; ▪) or presence of blocking mAb against αvβ3-integrin, LM609 (for VN and FBG), the blocking mAb against β1-integrin, 6S6 (for FN) (20 μg/mL; □), or increasing concentrations of Eap ( ) as indicated. Specific binding is expressed as absorbance at 405 nm. Data are means ± SD (n = 3) of a typical experiment; similar results were observed in 3 separate experiments.
) as indicated. Specific binding is expressed as absorbance at 405 nm. Data are means ± SD (n = 3) of a typical experiment; similar results were observed in 3 separate experiments.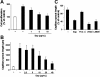
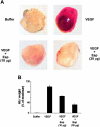
References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 2003;339: 520-532. - PubMed
-
- Waldvogel FA. New resistance in Staphylococcus aureus. N Engl J Med. 1999;340: 556-557. - PubMed
-
- Madsen SM, Westh H, Danielsen L, Rosdahl VT. Bacterial colonization and healing of venous leg ulcers. APMIS. 1996;104: 895-899. - PubMed
-
- Grimble SA, Magee TR, Galland RB. Methicillin resistant Staphylococcus aureus in patients undergoing major amputation. Eur J Vasc Endovasc Surg. 2001;22: 215-218. - PubMed
-
- Flock JI. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol Med Today. 1999;12: 532-537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

