Are racial and ethnic minorities less willing to participate in health research?
- PMID: 16318411
- PMCID: PMC1298944
- DOI: 10.1371/journal.pmed.0030019
Are racial and ethnic minorities less willing to participate in health research?
Abstract
Background: It is widely claimed that racial and ethnic minorities, especially in the US, are less willing than non-minority individuals to participate in health research. Yet, there is a paucity of empirical data to substantiate this claim.
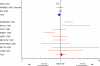
Methods and findings: We performed a comprehensive literature search to identify all published health research studies that report consent rates by race or ethnicity. We found 20 health research studies that reported consent rates by race or ethnicity. These 20 studies reported the enrollment decisions of over 70,000 individuals for a broad range of research, from interviews to drug treatment to surgical trials. Eighteen of the twenty studies were single-site studies conducted exclusively in the US or multi-site studies where the majority of sites (i.e., at least 2/3) were in the US. Of the remaining two studies, the Concorde study was conducted at 74 sites in the United Kingdom, Ireland, and France, while the Delta study was conducted at 152 sites in Europe and 23 sites in Australia and New Zealand. For the three interview or non-intervention studies, African-Americans had a nonsignificantly lower overall consent rate than non-Hispanic whites (82.2% versus 83.5%; odds ratio [OR] = 0.92; 95% confidence interval [CI] 0.84-1.02). For these same three studies, Hispanics had a nonsignificantly higher overall consent rate than non-Hispanic whites (86.1% versus 83.5%; OR = 1.37; 95% CI 0.94-1.98). For the ten clinical intervention studies, African-Americans' overall consent rate was nonsignificantly higher than that of non-Hispanic whites (45.3% versus 41.8%; OR = 1.06; 95% CI 0.78-1.45). For these same ten studies, Hispanics had a statistically significant higher overall consent rate than non-Hispanic whites (55.9% versus 41.8%; OR = 1.33; 95% CI 1.08-1.65). For the seven surgery trials, which report all minority groups together, minorities as a group had a nonsignificantly higher overall consent rate than non-Hispanic whites (65.8% versus 47.8%; OR = 1.26; 95% CI 0.89-1.77). Given the preponderance of US sites, the vast majority of these individuals from minority groups were African-Americans or Hispanics from the US.
Conclusions: We found very small differences in the willingness of minorities, most of whom were African-Americans and Hispanics in the US, to participate in health research compared to non-Hispanic whites. These findings, based on the research enrollment decisions of over 70,000 individuals, the vast majority from the US, suggest that racial and ethnic minorities in the US are as willing as non-Hispanic whites to participate in health research. Hence, efforts to increase minority participation in health research should focus on ensuring access to health research for all groups, rather than changing minority attitudes.
Conflict of interest statement
Figures


Comment in
-
Why are ethnic minorities under-represented in US research studies?PLoS Med. 2006 Feb;3(2):e49. doi: 10.1371/journal.pmed.0030049. Epub 2005 Dec 27. PLoS Med. 2006. PMID: 16370583 Free PMC article.
-
Mistrust among minorities and the trustworthiness of medicine.PLoS Med. 2006 May;3(5):e244; author reply e245. doi: 10.1371/journal.pmed.0030244. Epub 2006 May 30. PLoS Med. 2006. PMID: 16719549 Free PMC article. No abstract available.
References
-
- Hussain-Gambles M. Ethnic minority under-representation in clinical trials: Whose responsibility is it anyway? J Health Organ Manag. 2003;17:138–143. - PubMed
-
- Britton A, McKee M, Black N, McPherson K, Sanderson C, et al. Threats to applicability of randomized trials: Exclusion and selective participation. J Health Serv Res Policy. 1999;4:112–121. - PubMed
-
- Freedman LS, Simon R, Foulkes MA, Friedman L, Geller NL, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993—The perspective of NIH clinical trialists. Control Clin Trials. 1995;16:277–285. - PubMed
-
- Shavers VL, Lynch CF, Burmeister LF. Factors that influence African-Americans' willingness to participate in medical research studies. Cancer. 2001;91(Suppl):233–236. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

