Catalytic mechanism and energy barriers for butyrylcholinesterase-catalyzed hydrolysis of cocaine
- PMID: 16319079
- PMCID: PMC1366953
- DOI: 10.1529/biophysj.105.070276
Catalytic mechanism and energy barriers for butyrylcholinesterase-catalyzed hydrolysis of cocaine
Abstract
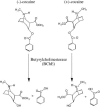
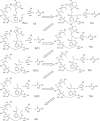
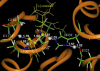
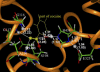
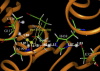
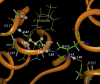
The geometries of the transition states, intermediates, and prereactive enzyme-substrate complex and the corresponding energy barriers have been determined by performing hybrid quantum mechanical/molecular mechanical (QM/MM) calculations on butyrylcholinesterase (BChE)-catalyzed hydrolysis of (-)- and (+)-cocaine. The energy barriers were evaluated by performing QM/MM calculations with the QM method at the MP2/6-31+G* level and the MM method using the AMBER force field. These calculations allow us to account for the protein environmental effects on the transition states and energy barriers of these enzymatic reactions, showing remarkable effects of the protein environment on intermolecular hydrogen bonding (with an oxyanion hole), which is crucial for the transition state stabilization and, therefore, on the energy barriers. The calculated energy barriers are consistent with available experimental kinetic data. The highest barrier calculated for BChE-catalyzed hydrolysis of (-)- and (+)-cocaine is associated with the third reaction step, but the energy barrier calculated for the first step is close to the highest and is so sensitive to the protein environment that the first reaction step can be rate determining for (-)-cocaine hydrolysis catalyzed by a BChE mutant. The computational results provide valuable insights into future design of BChE mutants with a higher catalytic activity for (-)-cocaine.
Figures



References
-
- Gawin, F. H., and E. H. Ellinwood Jr. 1988. Cocaine and other stimulants: actions, abuse, and treatment. N. Eng. J. Med. 318:1173–1182. - PubMed
-
- Landry, D. W. 1997. Immunotherapy for cocaine addiction. Sci. Am. 276:42–45. - PubMed
-
- Singh, S. 2000. Chemistry, design, and structure-activity relationship of cocaine antagonists. Chem. Rev. 100:925–1024. - PubMed
-
- Paula, S., M. R. Tabet, C. D. Farr, A. B. Norman, and W. J. Ball Jr. 2004. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J. Med. Chem. 47:133–142. - PubMed
-
- Sparenborg, S., F. Vocci, and S. Zukin. 1997. Peripheral cocaine-blocking agents: new medications for cocaine dependence: an introduction to immunological and enzymatic approaches to treating cocaine dependence reported by Fox, Gorelick and Cohen in the immediately succeeding articles. Drug Alcohol Depend. 48:149–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

