Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii
- PMID: 16319175
- PMCID: PMC1356598
- DOI: 10.1091/mbc.e05-06-0512
Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii
Abstract
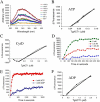
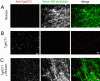
Toxoplasma is a protozoan parasite in the phylum Apicomplexa, which contains a number of medically important parasites that rely on a highly unusual form of motility termed gliding to actively penetrate their host cells. Parasite actin filaments regulate gliding motility, yet paradoxically filamentous actin is rarely detected in these parasites. To investigate the kinetics of this unusual parasite actin, we expressed TgACT1 in baculovirus and purified it to homogeneity. Biochemical analysis showed that Toxoplasma actin (TgACT1) rapidly polymerized into filaments at a critical concentration that was 3-4-fold lower than conventional actins, yet it failed to copolymerize with mammalian actin. Electron microscopic analysis revealed that TgACT1 filaments were 10 times shorter and less stable than rabbit actin. Phylogenetic comparison of actins revealed a limited number of apicomplexan-specific residues that likely govern the unusual behavior of parasite actin. Molecular modeling identified several key alterations that affect interactions between monomers and that are predicted to destabilize filaments. Our findings suggest that conserved molecular differences in parasite actin favor rapid cycles of assembly and disassembly that govern the unusual form of gliding motility utilized by apicomplexans.
Figures





References
-
- Allen, M. L., Dobrowolski, J. M., Muller, H., Sibley, L. D., and Mansour, T. E. (1997). Cloning and characterization of actin depolymerizing factor from Toxoplasma gondii. Mol. Biochem. Parasitol. 88, 43-52. - PubMed
-
- Baldauf, S. L., Roger, A. J., Wenk-Siefert, I., and Doolittle, W. F. (2000). A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290, 972-977. - PubMed
-
- Barragan, A., and Sibley, L. D. (2003). Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 11, 426-430. - PubMed
-
- Bubb, M. R., Senderowicz, A.M.J., Sausville, E. A., Duncan, K.L.K., and Korn, E. D. (1994). Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14869-14871. - PubMed
-
- Bubb, M. R., Spector, I., Beyer, B. B., and Fosen, K. M. (2000). Effects of jasplakinolide on the kinetics of actin polymerization. J. Biol. Chem. 275, 5163-5170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

