Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels
- PMID: 16319223
- PMCID: PMC1297712
- DOI: 10.1073/pnas.0509122102
Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels
Abstract
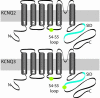
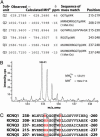
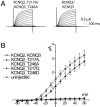
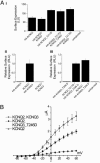
Neuronal potassium channel subunits of the KCNQ (Kv7) family underlie M-current (I(M)), and may also underlie the slow potassium current at the node of Ranvier, I(Ks). I(M) and I(Ks) are outwardly rectifying currents that regulate excitability of neurons and myelinated axons, respectively. Studies of native I(M) and heterologously expressed Kv7 subunits suggest that, in vivo, KCNQ channels exist within heterogeneous, multicomponent protein complexes. KCNQ channel properties are regulated by protein phosphorylation, protein-protein interactions, and protein-lipid interactions within such complexes. To better understand the regulation of neuronal KCNQ channels, we searched directly for posttranslational modifications on KCNQ2/KCNQ3 channels in vivo by using mass spectrometry. Here we describe two sites of phosphorylation. One site, specific for KCNQ3, appears functionally silent in electrophysiological assays but is located in a domain previously shown to be important for subunit tetramerization. Mutagenesis and electrophysiological studies of the second site, located in the S4-S5 intracellular loop of all KCNQ subunits, reveal a mechanism of channel inhibition.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

