Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling
- PMID: 16319315
- PMCID: PMC6725650
- DOI: 10.1523/JNEUROSCI.3821-05.2005
Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling
Abstract
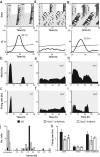
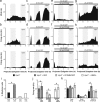
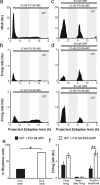
Vasoactive intestinal polypeptide (VIP) and gastrin-releasing peptide (GRP) acting via the VPAC2 receptor and BB2 receptors, respectively, are key signaling pathways in the suprachiasmatic nuclei (SCN) circadian clock. Transgenic mice lacking the VPAC2 receptor (Vipr2(-/-)) display a continuum of disrupted behavioral rhythms with only a minority capable of sustaining predictable cycles of rest and activity. However, electrical or molecular oscillations have not yet been detected in SCN cells from adult Vipr2(-/-) mice. Using a novel electrophysiological recording technique, we found that in brain slices from wild-type and behaviorally rhythmic Vipr2(-/-) mice, the majority of SCN neurons we detected displayed circadian firing patterns with estimated periods similar to the animals' behavior. In contrast, in slices from behaviorally arrhythmic Vipr2(-/-) mice, only a small minority of the observed SCN cells oscillated. Remarkably, exogenous GRP promoted SCN cellular rhythms in Vipr2(-/-) mouse slices, whereas blockade of BB2 receptors suppressed neuronal oscillations. In wild-type mice, perturbation of GRP-BB2 signaling had few effects on SCN cellular rhythms, except when VPAC2 receptors were blocked pharmacologically. These findings establish that residual electrical oscillations persist in the SCN of Vipr2(-/-) mice and reveal a potential new role for GRP-BB2 signaling within the circadian clock.
Figures


References
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S (2002) Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61: 26-34. - PubMed
-
- Aioun J, Chambille I, Peytevin J, Martinet L (1998) Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res 291: 239-253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
