Lipid-protein interactions in double-layered two-dimensional AQP0 crystals
- PMID: 16319884
- PMCID: PMC1350984
- DOI: 10.1038/nature04321
Lipid-protein interactions in double-layered two-dimensional AQP0 crystals
Erratum in
- Nature. 2006 May 11;441(7090):248
Abstract
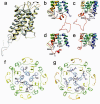
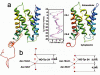
Lens-specific aquaporin-0 (AQP0) functions as a specific water pore and forms the thin junctions between fibre cells. Here we describe a 1.9 A resolution structure of junctional AQP0, determined by electron crystallography of double-layered two-dimensional crystals. Comparison of junctional and non-junctional AQP0 structures shows that junction formation depends on a conformational switch in an extracellular loop, which may result from cleavage of the cytoplasmic amino and carboxy termini. In the centre of the water pathway, the closed pore in junctional AQP0 retains only three water molecules, which are too widely spaced to form hydrogen bonds with each other. Packing interactions between AQP0 tetramers in the crystalline array are mediated by lipid molecules, which assume preferred conformations. We were therefore able to build an atomic model for the lipid bilayer surrounding the AQP0 tetramers, and we describe lipid-protein interactions.
Figures


Comment in
-
Cell biology: a greasy grip.Nature. 2005 Dec 1;438(7068):569-70. doi: 10.1038/438569a. Nature. 2005. PMID: 16319869 No abstract available.
References
-
- Murata K, et al. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. - PubMed
-
- Fu D, et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. - PubMed
-
- Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

