NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation
- PMID: 16319923
- PMCID: PMC1356344
- DOI: 10.1038/sj.emboj.7600900
NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation
Abstract
Cells latently infected with HIV represent a currently insurmountable barrier to viral eradication in infected patients. Using the J-Lat human T-cell model of HIV latency, we have investigated the role of host factor binding to the kappaB enhancer elements of the HIV long terminal repeat (LTR) in the maintenance of viral latency. We show that NF-kappaB p50-HDAC1 complexes constitutively bind the latent HIV LTR and induce histone deacetylation and repressive changes in chromatin structure of the HIV LTR, changes that impair recruitment of RNA polymerase II and transcriptional initiation. Knockdown of p50 expression with specific small hairpin RNAs reduces HDAC1 binding to the latent HIV LTR and induces RNA polymerase II recruitment. Similarly, inhibition of histone deacetylase (HDAC) activity with trichostatin A promotes binding of RNA polymerase II to the latent HIV LTR. This bound polymerase complex, however, remains non-processive, generating only short viral transcripts. Synthesis of full-length viral transcripts can be rescued under these conditions by expression of Tat. The combination of HDAC inhibitors and Tat merits consideration as a new strategy for purging latent HIV proviruses from their cellular reservoirs.
Figures
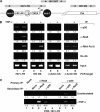
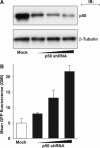



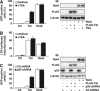
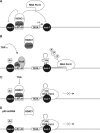
References
-
- Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG (2002) Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell 110: 55–67 - PubMed
-
- Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM (2001) NF-kappaB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol Cell 8: 327–337 - PubMed
-
- Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653–1657 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

