Quality control of protein folding in extracellular space
- PMID: 16319958
- PMCID: PMC1369217
- DOI: 10.1038/sj.embor.7400586
Quality control of protein folding in extracellular space
Abstract
The pathologies of many serious human diseases are thought to develop from the effects of intra- or extracellular aggregates of non-native proteins. Inside cells, chaperone and protease systems regulate protein folding; however, little is known about any corresponding mechanisms that operate extracellularly. The identification of these mechanisms is important for the development of new disease therapies. This review briefly discusses the consequences of protein misfolding, the intracellular mechanisms that control folding and the potential corresponding extracellular control processes. Finally, a new speculative model is described, which proposes that newly discovered extracellular chaperones bind to exposed regions of hydrophobicity on non-native, extracellular proteins to target them for receptor-mediated endocytosis and intracellular, lysosomal degradation.
Figures

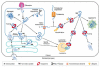

References
-
- Akarsu E, Pirim I, Selcuk NY, Tombul HZ, Cetinkaya R (2001) Relation between serum ubiquitin levels and KT/V in chronic hemodialysis patients. Nephron 88: 280–282 - PubMed
-
- Bajari TM, Strasser V, Nimpf J, Schneider WJ (2003) A model for modulation of leptin activity by association with clusterin. FASEB J 17: 1505–1507 - PubMed
-
- Bartl MM, Luckenbach T, Bergner O, Ullrich O, Koch-Brandt C (2001) Multiple receptors mediate apoJ-dependent clearance of cellular debris into nonprofessional phagocytes. Exp Cell Res 271: 130–141 - PubMed
-
- Born LJ Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM Jr (1996) Effects of ethanol administration on components of the ubiquitin proteolytic pathway in rat liver. Hepatol 23: 1556–1563 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

