Identification of polyclonal and monoclonal antibodies against tissue plasminogen activator in the antiphospholipid syndrome
- PMID: 16320350
- PMCID: PMC1950581
- DOI: 10.1002/art.21485
Identification of polyclonal and monoclonal antibodies against tissue plasminogen activator in the antiphospholipid syndrome
Abstract
Objective: To test the hypotheses that some plasmin-reactive anticardiolipin antibodies (aCL) may bind to tissue plasminogen activator (tPA) and that some of the tPA-reactive aCL may inhibit tPA activity.
Methods: We studied the reactivity of 8 patient-derived monoclonal aCL with tPA and examined the presence of IgG anti-tPA antibodies in patients with the antiphospholipid syndrome (APS). The effects of the reactive monoclonal aCL on the activity of tPA were also examined.
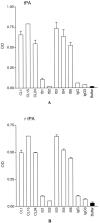
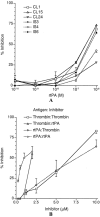
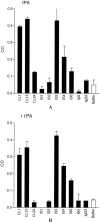
Results: Six patient-derived plasmin-reactive monoclonal aCL bound to tPA. Analysis of plasma samples revealed that 10 of 80 APS patients (12.5%) and 1 of 81 systemic lupus erythematosus patients (1.2%) had antibodies against fibrin-associated tPA, based on a cutoff value equal to the mean + 2SD of the level in 28 normal subjects. Of the 6 tPA-reactive monoclonal aCL, 2 of them (CL1 and CL15) inhibited tPA activity.
Conclusion: Some of the plasmin-reactive aCL in APS patients may bind to tPA. Of the tPA-reactive aCL, some (such as CL1 and CL15) may inhibit tPA activity and, thus, may be prothrombotic in the host.
Figures


References
-
- Harris EN, Pierangeli SS. Antiphospholipid antibodies and the antiphospholipid syndrome. Springer Semin Immunopathol. 1994;16:223–45. - PubMed
-
- Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet. 1990;336:177–8. - PubMed
-
- Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJC, Barbui T, Zwaal RFA, Bevers EM. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–7. - PubMed
-
- Fleck RA, Rapaport SI, Rao LV. Anti-prothrombin antibodies and the lupus anticoagulant. Blood. 1988;72:512–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

