Consequences of the electrogenic function of the phagocytic NADPH oxidase
- PMID: 16321799
- PMCID: PMC1569590
- DOI: 10.1098/rstb.2005.1768
Consequences of the electrogenic function of the phagocytic NADPH oxidase
Abstract
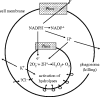
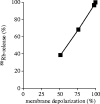
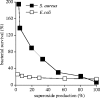
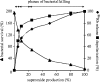
NADPH oxidase of phagocytic cells transfers a single electron from intracellular NADPH to extracellular O2, producing superoxide (O.-2), the precursor to several other reactive oxygen species. The finding that a genetic defect of the enzyme causes chronic granulomatous disease (CGD), characterized by recurrent severe bacterial infections, linked O.-2 generation to destruction of potentially pathogenic micro-organisms. In this review, we focus on the consequences of the electrogenic functioning of NADPH oxidase. We show that enzyme activity depends on the possibilities for compensating charge movements. In resting neutrophils K+ conductance dominates, but upon activation the plasma membrane rapidly depolarizes beyond the opening threshold of voltage-gated H+ channels and H+ efflux becomes the major charge compensating factor. K+ release is likely to contribute to the killing of certain bacteria but complete elimination only occurs if O.-2 production can proceed at full capacity. Finally, the reversed membrane potential of activated neutrophils inhibits Ca2+ entry, thereby preventing overloading the cells with Ca2+. Absence of this limiting mechanism in CGD cells may contribute to the pathogenesis of the disease.
Figures
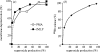
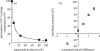
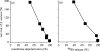
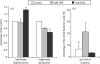
References
-
- DeCoursey T.E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003;83:475–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
