Oxidative stress and ageing: is ageing a cysteine deficiency syndrome?
- PMID: 16321806
- PMCID: PMC1569588
- DOI: 10.1098/rstb.2005.1770
Oxidative stress and ageing: is ageing a cysteine deficiency syndrome?
Abstract
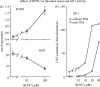
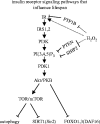
Reactive oxygen species (ROS) are constantly produced in biological tissues and play a role in various signalling pathways. Abnormally high ROS concentrations cause oxidative stress associated with tissue damage and dysregulation of physiological signals. There is growing evidence that oxidative stress increases with age. It has also been shown that the life span of worms, flies and mice can be significantly increased by mutations which impede the insulin receptor signalling cascade. Molecular studies revealed that the insulin-independent basal activity of the insulin receptor is increased by ROS and downregulated by certain antioxidants. Complementary clinical studies confirmed that supplementation of the glutathione precursor cysteine decreases insulin responsiveness in the fasted state. In several clinical trials, cysteine supplementation improved skeletal muscle functions, decreased the body fat/lean body mass ratio, decreased plasma levels of the inflammatory cytokine tumour necrosis factor alpha (TNF-alpha), improved immune functions, and increased plasma albumin levels. As all these parameters degenerate with age, these findings suggest: (i) that loss of youth, health and quality of life may be partly explained by a deficit in cysteine and (ii) that the dietary consumption of cysteine is generally suboptimal and everybody is likely to have a cysteine deficiency sooner or later.
Figures




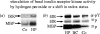
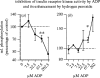
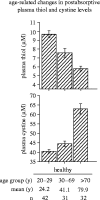

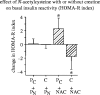
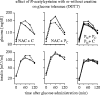

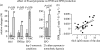
References
-
- Azzoni L, Papasavvas E, Chehimi J, Kostman J.R, Mounzer K, Ondercin J, Perussia B, Montaner L.J. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 2002;168:5764–5770. - PubMed
-
- Balkan J, Kanbagli O, Mehmetcik G, Mutlu-Turkoglu U, Aykac-Toker G, Uysal M. Increased lipid peroxidation in serum and low-density lipoproteins associated with aging in humans. Int. J. Vitam. Nutr. Res. 2002;72:315–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
