The maltodextrin system of Escherichia coli: metabolism and transport
- PMID: 16321936
- PMCID: PMC1316994
- DOI: 10.1128/JB.187.24.8322-8331.2005
The maltodextrin system of Escherichia coli: metabolism and transport
Abstract
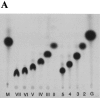
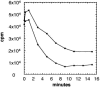
The maltose/maltodextrin regulon of Escherichia coli consists of 10 genes which encode a binding protein-dependent ABC transporter and four enzymes acting on maltodextrins. All mal genes are controlled by MalT, a transcriptional activator that is exclusively activated by maltotriose. By the action of amylomaltase, we prepared uniformly labeled [(14)C]maltodextrins from maltose up to maltoheptaose with identical specific radioactivities with respect to their glucosyl residues, which made it possible to quantitatively follow the rate of transport for each maltodextrin. Isogenic malQ mutants lacking maltodextrin phosphorylase (MalP) or maltodextrin glucosidase (MalZ) or both were constructed. The resulting in vivo pattern of maltodextrin metabolism was determined by analyzing accumulated [(14)C]maltodextrins. MalP(-) MalZ(+) strains degraded all dextrins to maltose, whereas MalP(+) MalZ(-) strains degraded them to maltotriose. The labeled dextrins were used to measure the rate of transport in the absence of cytoplasmic metabolism. Irrespective of the length of the dextrin, the rates of transport at a submicromolar concentration were similar for the maltodextrins when the rate was calculated per glucosyl residue, suggesting a novel mode for substrate translocation. Strains lacking MalQ and maltose transacetylase were tested for their ability to accumulate maltose. At 1.8 nM external maltose, the ratio of internal to external maltose concentration under equilibrium conditions reached 10(6) to 1 but declined at higher external maltose concentrations. The maximal internal level of maltose at increasing external maltose concentrations was around 100 mM. A strain lacking malQ, malP, and malZ as well as glycogen synthesis and in which maltodextrins are not chemically altered could be induced by external maltose as well as by all other maltodextrins, demonstrating the role of transport per se for induction.
Figures



References
-
- Bavoil, P., M. Hofnung, and H. Nikaido. 1980. Identification of a cytoplasmic membrane-associated component of the maltose transport system of Escherichia coli. J. Biol. Chem. 255:8366-8369. - PubMed
-
- Böhm, A., J. Diez, K. Diederichs, W. Welte, and W. Boos. 2002. Structural model of MalK, the ABC subunit of the maltose transporter of Escherichia coli. Implications for mal gene regulation, inducer exclusion, and subunit assembly. J. Biol. Chem. 277:3708-3717. - PubMed
-
- Boos, W., and J. M. Lucht. 1996. Periplasmic binding protein-dependent ABC transporters, p. 1175-1209. In F. C. Neidhardt, R. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

