The maltodextrin system of Escherichia coli: glycogen-derived endogenous induction and osmoregulation
- PMID: 16321937
- PMCID: PMC1316995
- DOI: 10.1128/JB.187.24.8332-8339.2005
The maltodextrin system of Escherichia coli: glycogen-derived endogenous induction and osmoregulation
Abstract
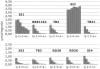
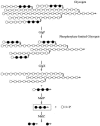
Strains of Escherichia coli lacking MalQ (maltodextrin glucanotransferase or amylomaltase) are endogenously induced for the maltose regulon by maltotriose that is derived from the degradation of glycogen (glycogen-dependent endogenous induction). A high level of induction was dependent on the presence of MalP, maltodextrin phosphorylase, while expression was counteracted by MalZ, maltodextrin glucosidase. Glycogen-derived endogenous induction was sensitive to high osmolarity. This osmodependence was caused by MalZ. malZ, the gene encoding this enzyme, was found to be induced by high osmolarity even in the absence of MalT, the central regulator of all mal genes. The osmodependent expression of malZ was neither RpoS nor OmpR dependent. In contrast, the malPQ operon, whose expression was also increased at a high osmolarity, was partially dependent on RpoS. In the absence of glycogen, residual endogenous induction of the mal genes that is sensitive to increasing osmolarity can still be observed. This glycogen-independent endogenous induction is not understood, and it is not affected by altering the expression of MalP, MalQ, and MalZ. In particular, its independence from MalZ suggests that the responsible inducer is not maltotriose.
Figures
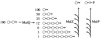
References
-
- Becker, G., and R. Hengge-Aronis. 2001. What makes an Escherichia coli promoter σS dependent? Role of the −13/−14 nucleotide promoter positions and region 2.5 of σS. Mol. Microbiol. 39:1153-1165. - PubMed
-
- Becker, G., E. Klauck, and R. Hengge-Aronis. 2000. The response regulator RssB, a recognition factor for σS proteolysis in Escherichia coli, can act like an anti-σS factor. Mol. Microbiol. 35:657-666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

