Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells
- PMID: 16322055
- PMCID: PMC1805644
- DOI: 10.1113/jphysiol.2005.099135
Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells
Abstract
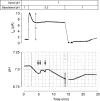
Renal net acid excretion requires tubular reabsorption of filtered bicarbonate, followed by secretion of protons and ammonium in the collecting duct, generating steep transtubular gradients for these ions. To prevent passive backleak of these ions, the tight junctions in the collecting duct must be highly impermeable to these ions. We previously generated a Madin-Darby canine kidney (MDCK II) cell line with inducible expression of claudin-8, a tight junction protein expressed in the collecting duct. In these cells, claudin-8 was shown to function as a paracellular barrier to alkali metal and divalent cations. We have now used this model to test the hypothesis that claudin-8 also functions as a paracellular barrier to acidic or basic ions involved in renal acid excretion. We developed a series of precise and unbiased methods, based on a combination of diffusion potential, short-circuit current, and pH stat measurements, to estimate paracellular permeability to protons, ammonium and bicarbonate in MDCK II cells. We found that under control conditions (i.e. in the absence of claudin-8), these cells are highly permeable to the acidic and basic ions tested. Interestingly, proton permeation exhibited an unusually low activation energy similar to that in bulk solution. This suggests that paracellular proton transfer may occur by a Grotthuss mechanism, implying that the paracellular pores are sufficiently wide to accommodate water molecules in a freely mobile state. Induction of claudin-8 expression reduces permeability not only to protons, but also to ammonium and bicarbonate. We conclude that claudin-8 probably functions to limit the passive leak of these three ions via paracellular routes, thereby playing a permissive role in urinary net acid excretion.
Figures

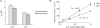


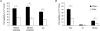


References
-
- Adson A, Raub TJ, Burton PS, Barsuhn CL, Hilgers AR, Audus KL, Ho NF. Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. J Pharm Sci. 1994;83:1529–1536. - PubMed
-
- Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–2693. - PubMed
-
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. - PubMed
-
- Batlle D, Flores G. Underlying defects in distal renal tubular acidosis: New understandings. Am J Kid Dis. 1996;27:896–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

