Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes
- PMID: 16322346
- PMCID: PMC1402357
- DOI: 10.1152/ajpregu.00734.2005
Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes
Abstract
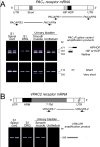
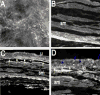
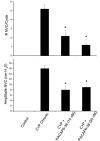
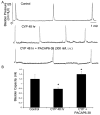
Pituitary adenylate cyclase activating polypeptide (PACAP) peptides are expressed and regulated in sensory afferents of the micturition pathway. Although these studies have implicated PACAP in bladder control, the physiological significance of these observations has not been firmly established. To clarify these issues, the roles of PACAP and PACAP signaling in micturition and cystitis were examined in receptor characterization and physiological assays. PACAP receptors were identified in various tissues of the micturition pathway, including bladder detrusor smooth muscle and urothelium. Bladder smooth muscle expressed heterogeneously PAC(1)null, PAC(1)HOP1, and VPAC(2) receptors; the urothelium was more restricted in expressing preferentially the PAC(1) receptor subtype only. Immunocytochemical studies for PAC(1) receptors were consistent with these tissue distributions. Furthermore, the addition of 50-100 nM PACAP27 or PACAP38 to isolated bladder strips elicited transient contractions and sustained increases in the amplitude of spontaneous phasic contractions. Treatment of the bladder strips with tetrodotoxin (1 muM) did not alter the spontaneous phasic contractions suggesting direct PACAP effects on bladder smooth muscle. PACAP also increased the amplitude of nerve-evoked contractions. By contrast, vasoactive intestinal polypeptide had no direct effects on bladder smooth muscle. In a rat cyclophosphamide (CYP)-induced cystitis paradigm, intrathecal or intravesical administration of PAC(1) receptor antagonist, PACAP6-38, reduced cystitis-induced bladder overactivity. In summary, these studies support roles for PACAP in micturition and suggest that inflammation-induced plasticity in PACAP expression in peripheral and central micturition pathways contribute to bladder dysfunction with cystitis.
Figures


References
-
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. - PubMed
-
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. - PubMed
-
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–1091. - PubMed
-
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

