Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver
- PMID: 16322469
- PMCID: PMC1895379
- DOI: 10.1182/blood-2005-10-4035
Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver
Abstract
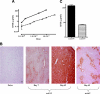
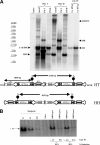
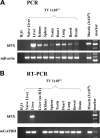
Transduction with recombinant adeno-associated virus (AAV) vectors is limited by the need to convert its single-stranded (ss) genome to transcriptionally active double-stranded (ds) forms. For AAV-mediated hemophilia B (HB) gene therapy, we have overcome this obstacle by constructing a liver-restricted mini-human factor IX (hFIX) expression cassette that can be packaged as complementary dimers within individual AAV particles. Molecular analysis of murine liver transduced with these self-complementary (sc) vectors demonstrated rapid formation of active ds-linear genomes that persisted stably as concatamers or monomeric circles. This unique property resulted in a 20-fold improvement in hFIX expression in mice over comparable ssAAV vectors. Administration of only 1 x 10(10) scAAV particles led to expression of hFIX at supraphysiologic levels (8I U/mL) and correction of the bleeding diathesis in FIX knock-out mice. Of importance, therapeutic levels of hFIX (3%-30% of normal) were achieved in nonhuman primates using a significantly lower dose of scAAV than required with ssAAV. Furthermore, AAV5-pseudotyped scAAV vectors mediated successful transduction in macaques with pre-existing immunity to AAV8. Hence, this novel vector represents an important advance for hemophilia B gene therapy.
Figures

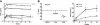
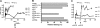
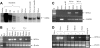
Comment in
-
Hemophilia B gene therapy in humans shows promise.Circ Cardiovasc Genet. 2012 Apr 1;5(2):269-70. doi: 10.1161/CIRCGENETICS.112.963231. Circ Cardiovasc Genet. 2012. PMID: 22511710 No abstract available.
References
-
- Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5: 383-439. - PubMed
-
- Nathwani AC, Davidoff AM, Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10: 309-318. - PubMed
-
- Nakai H, Storm TA, Kay MA. Annealing of complimentary single stranded genomes and subsequent intermolecular joining is the mechanism of stable in vivo liver transduction by recombinant adeno-associated virus vectors [abstract]. Mol Ther. 2000;1: S125-S126.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

