De novo GMP synthesis is required for axon guidance in Drosophila
- PMID: 16322525
- PMCID: PMC1456273
- DOI: 10.1534/genetics.105.042911
De novo GMP synthesis is required for axon guidance in Drosophila
Abstract
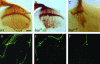
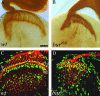
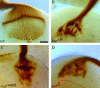
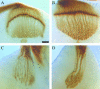
Guanine nucleotides are key players in mediating growth-cone signaling during neural development. The supply of cellular guanine nucleotides in animals can be achieved via the de novo synthesis and salvage pathways. The de novo synthesis of guanine nucleotides is required for lymphocyte proliferation in animals. Whether the de novo synthesis pathway is essential for any other cellular processes, however, remains unknown. In a search for genes required for the establishment of neuronal connectivity in the fly visual system, we identify the burgundy (bur) gene as an essential player in photoreceptor axon guidance. The bur gene encodes the only GMP synthetase in Drosophila that catalyzes the final reaction of de novo GMP synthesis. Loss of bur causes severe defects in axonal fasciculation, retinotopy, and growth-cone morphology, but does not affect photoreceptor differentiation or retinal patterning. Similar defects were observed when the raspberry (ras) gene, encoding for inosine monophosphate dehydrogenase catalyzing the IMP-to-XMP conversion in GMP de novo synthesis, was mutated. Our study thus provides the first in vivo evidence to support an essential and specific role for de novo synthesis of guanine nucleotides in axon guidance.
Figures




References
-
- Bork, P., and E. V. Koonin, 1994. A P-loop-like motif in a widespread ATP pyrophosphatase domain: implications for the evolution of sequence motifs and enzyme activity. Proteins 20: 347–355. - PubMed
-
- Carthew, R. W., and G. M. Rubin, 1990. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell 63: 561–577. - PubMed
-
- Clandinin, T. R., and S. L. Zipursky, 2002. Making connections in the fly visual system. Neuron 35: 827–841. - PubMed
-
- Dayton, J. S., L. A. Turka, C. B. Thompson and B. S. Mitchell, 1992. Comparison of the effects of mizoribine with those of azathioprine, 6-mercaptopurine, and mycophenolic acid on T lymphocyte proliferation and purine ribonucleotide metabolism. Mol. Pharmacol. 41: 671–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

