Immunity by ubiquitylation: a reversible process of modification
- PMID: 16322747
- PMCID: PMC7096784
- DOI: 10.1038/nri1731
Immunity by ubiquitylation: a reversible process of modification
Abstract
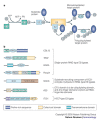
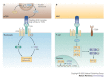
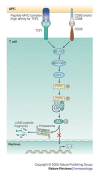
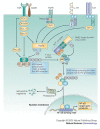
The conjugation of ubiquitin, a 76-amino-acid peptide, to a protein substrate provides a tag that either marks the labelled protein for degradation or modulates its function. The process of protein ubiquitylation--which is catalysed by coordinated enzymatic reactions that are mediated by enzymes known as E1, E2 and E3--has an important role in the modulation of immune responses. Importantly, protein ubiquitylation is a reversible process, and removal of ubiquitin molecules is mediated by de-ubiquitylating enzymes: for example, A20, which has been implicated in the regulation of immune responses. In addition, the conjugation of ubiquitin-like molecules, such as ISG15 (interferon-stimulated protein of 15 kDa), to proteins is also involved in immune regulation. This Review covers recent progress in our understanding of protein ubiquitylation in the immune system.
Conflict of interest statement
Josef Penninger holds shares in a company that plans to make CBL-B inhibitors.
Figures
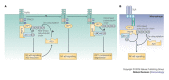
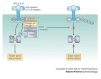
References
-
- Pickart CM. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. - PubMed
-
- Weissman AM. Themes and variations on ubiquitylation. Nature Rev. Mol. Cell Biol. 2001;2:169–178. - PubMed
-
- Liu YC. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 2004;22:81–127. - PubMed
-
- Hicke L. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol. 2001;2:195–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

