Thrombus formation in vivo
- PMID: 16322780
- PMCID: PMC1297262
- DOI: 10.1172/JCI26987
Thrombus formation in vivo
Abstract
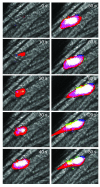
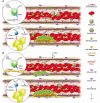
To examine thrombus formation in a living mouse, new technologies involving intravital videomicroscopy have been applied to the analysis of vascular windows to directly visualize arterioles and venules. After vessel wall injury in the microcirculation, thrombus development can be imaged in real time. These systems have been used to explore the role of platelets, blood coagulation proteins, endothelium, and the vessel wall during thrombus formation. The study of biochemistry and cell biology in a living animal offers new understanding of physiology and pathology in complex biologic systems.
Figures

References
-
- Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. - PubMed
-
- Ruggeri ZM. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. - PubMed
-
- Goto S. Understanding the mechanism of platelet thrombus formation under blood flow conditions and the effect of new antiplatelet agents. Curr. Vasc. Pharmacol. 2004;2:23–32. - PubMed
-
- Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb. Res. 2004;114:447–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

