Structure and function of the platelet integrin alphaIIbbeta3
- PMID: 16322781
- PMCID: PMC1297263
- DOI: 10.1172/JCI26989
Structure and function of the platelet integrin alphaIIbbeta3
Abstract
The platelet integrin alpha(IIb)beta(3) is required for platelet aggregation. Like other integrins, alpha(IIb)beta(3) resides on cell surfaces in an equilibrium between inactive and active conformations. Recent experiments suggest that the shift between these conformations involves a global reorganization of the alpha(IIb)beta(3) molecule and disruption of constraints imposed by the heteromeric association of the alpha(IIb) and beta(3) transmembrane and cytoplasmic domains. The biochemical, biophysical, and ultrastructural results that support this conclusion are discussed in this Review.
Figures


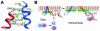


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

