Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays
- PMID: 16322790
- PMCID: PMC1297234
- DOI: 10.1172/JCI23587
Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays
Erratum in
- J Clin Invest. 2006 Feb;116(2):548. Zhen, Quan Li [corrected to Li, Quan-Zhen]
Abstract
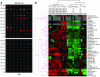
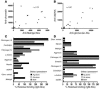
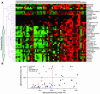
Nephrophilic autoantibodies dominate the seroprofile in lupus, but their fine specificities remain ill defined. We constructed a multiplexed proteome microarray bearing about 30 antigens known to be expressed in the glomerular milieu and used it to study serum autoantibodies in lupus. Compared with normal serum, serum from B6.Sle1.lpr lupus mice (C57BL/6 mice homozygous for the NZM2410/NZW allele of Sle1 as well as the FAS defect) exhibited high levels of IgG and IgM antiglomerular as well as anti-double-stranded DNA/chromatin Abs and variable levels of Abs to alpha-actinin, aggrecan, collagen, entactin, fibrinogen, hemocyanin, heparan sulphate, laminin, myosin, proteoglycans, and histones. The use of these glomerular proteome arrays also revealed 5 distinct clusters of IgG autoreactivity in the sera of lupus patients. Whereas 2 of these IgG reactivity clusters (DNA/chromatin/glomeruli and laminin/myosin/Matrigel/vimentin/heparan sulphate) showed association with disease activity, the other 3 reactivity clusters (histones, vitronectin/collagen/chondroitin sulphate, and entactin/fibrinogen/hyaluronic acid) did not. Human lupus sera also displayed 2 distinct IgM autoantibody clusters, one reactive to DNA and the other apparently polyreactive. Interestingly, the presence of IgM polyreactivity in patient sera was associated with reduced disease severity. Hence, the glomerular proteome array promises to be a powerful analytical tool for uncovering novel autoantibody disease associations and for distinguishing patients at high risk for end-organ disease.
Figures




References
-
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. - PubMed
-
- Hahn BH. Antibodies to DNA. N. Engl. J. Med. 1998;338:1359–1368. - PubMed
-
- Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus. Current concepts and continuing controversies. Arthritis Rheum. 1996;39:894–903. - PubMed
-
- Foster MH, Kelley VR. Lupus nephritis: update on pathogenesis and disease mechanisms. Semin. Nephrol. 1999;19:173–181. - PubMed
-
- Madaio MP, et al. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J. lmmunol. 1987;138:2883–2889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

