Antimicrobial activity of flavonoids
- PMID: 16323269
- PMCID: PMC7127073
- DOI: 10.1016/j.ijantimicag.2005.09.002
Antimicrobial activity of flavonoids
Erratum in
- Int J Antimicrob Agents. 2006 Feb;27(2):181
Abstract
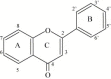
Flavonoids are ubiquitous in photosynthesising cells and are commonly found in fruit, vegetables, nuts, seeds, stems, flowers, tea, wine, propolis and honey. For centuries, preparations containing these compounds as the principal physiologically active constituents have been used to treat human diseases. Increasingly, this class of natural products is becoming the subject of anti-infective research, and many groups have isolated and identified the structures of flavonoids possessing antifungal, antiviral and antibacterial activity. Moreover, several groups have demonstrated synergy between active flavonoids as well as between flavonoids and existing chemotherapeutics. Reports of activity in the field of antibacterial flavonoid research are widely conflicting, probably owing to inter- and intra-assay variation in susceptibility testing. However, several high-quality investigations have examined the relationship between flavonoid structure and antibacterial activity and these are in close agreement. In addition, numerous research groups have sought to elucidate the antibacterial mechanisms of action of selected flavonoids. The activity of quercetin, for example, has been at least partially attributed to inhibition of DNA gyrase. It has also been proposed that sophoraflavone G and (-)-epigallocatechin gallate inhibit cytoplasmic membrane function, and that licochalcones A and C inhibit energy metabolism. Other flavonoids whose mechanisms of action have been investigated include robinetin, myricetin, apigenin, rutin, galangin, 2,4,2'-trihydroxy-5'-methylchalcone and lonchocarpol A. These compounds represent novel leads, and future studies may allow the development of a pharmacologically acceptable antimicrobial agent or class of agents.
Figures

References
-
- Infectious Diseases Society of America. Statement of the IDSA concerning ‘Bioshield II: Responding to an ever-changing threat’. Alexandria, VA: IDSA; 2004.
-
- Adcock H. Pharmageddon: is it too late to tackle growing resistance to anti-infectives? Pharm J. 2002;269:599–600.
-
- Jeu L., Piacenti F.J., Lyakhovetskiy A.G., Fung H.B. Voriconazole. Clin Ther. 2003;25:1321–1381. - PubMed
-
- De Clercq E. New developments in anti-HIV chemotherapy. Farmaco. 2001;56:3–12. - PubMed
-
- Poole K. Overcoming antimicrobial resistance by targeting resistance mechanisms. J Pharm Pharmacol. 2001;53:283–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

