Deconstructing the cadherin-catenin-actin complex
- PMID: 16325582
- PMCID: PMC3368712
- DOI: 10.1016/j.cell.2005.09.020
Deconstructing the cadherin-catenin-actin complex
Abstract
Spatial and functional organization of cells in tissues is determined by cell-cell adhesion, thought to be initiated through trans-interactions between extracellular domains of the cadherin family of adhesion proteins, and strengthened by linkage to the actin cytoskeleton. Prevailing dogma is that cadherins are linked to the actin cytoskeleton through beta-catenin and alpha-catenin, although the quaternary complex has never been demonstrated. We test this hypothesis and find that alpha-catenin does not interact with actin filaments and the E-cadherin-beta-catenin complex simultaneously, even in the presence of the actin binding proteins vinculin and alpha-actinin, either in solution or on isolated cadherin-containing membranes. Direct analysis in polarized cells shows that mobilities of E-cadherin, beta-catenin, and alpha-catenin are similar, regardless of the dynamic state of actin assembly, whereas actin and several actin binding proteins have higher mobilities. These results suggest that the linkage between the cadherin-catenin complex and actin filaments is more dynamic than previously appreciated.
Figures

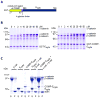
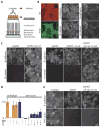

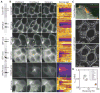
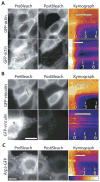
Comment in
-
Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions.Cell. 2005 Dec 2;123(5):769-72. doi: 10.1016/j.cell.2005.11.009. Cell. 2005. PMID: 16325573 Review.
References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
-
- Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002;115:4743–4753. - PubMed
-
- Cramer LP. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9:1095–1105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

