Universal behavior of membranes with sterols
- PMID: 16326903
- PMCID: PMC1367315
- DOI: 10.1529/biophysj.105.067652
Universal behavior of membranes with sterols
Abstract
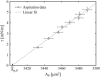
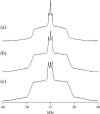
Lanosterol is the biosynthetic precursor of cholesterol and ergosterol, sterols that predominate in the membranes of mammals and lower eukaryotes, respectively. These three sterols are structurally quite similar, yet their relative effects on membranes have been shown to differ. Here we study the effects of cholesterol, lanosterol, and ergosterol on 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine lipid bilayers at room temperature. Micropipette aspiration is used to determine membrane material properties (area compressibility modulus), and information about lipid chain order (first moments) is obtained from deuterium nuclear magnetic resonance. We compare these results, along with data for membrane-bending rigidity, to explore the relationship between membrane hydrophobic thickness and elastic properties. Together, such diverse approaches demonstrate that membrane properties are affected to different degrees by these structurally distinct sterols, yet nonetheless exhibit universal behavior.
Figures

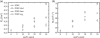

References
-
- Barenholz, Y. 2004. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. In Subcellular Biochemistry. P.J. Quinn, editor. Kluwer Academic/Plenum, New York. 167–215. - PubMed
-
- Simons, K., and W. L. C. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269–295. - PubMed
-
- Bloch, K. 1976. On the evolution of a biosynthetic pathway. In Reflections on Biochemistry. A. Kornberg, B.L. Horecker, L. Cornudella, and J. Orci, editors. Pergamon Press, New York. 143–150.
-
- Bloch, K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 19:47–92. - PubMed
-
- Nes, W. R. 1974. Role of sterols in membranes. Lipids. 9:596–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

