The Notch signaling pathway is related to neurovascular progression of pancreatic cancer
- PMID: 16327489
- PMCID: PMC1409885
- DOI: 10.1097/01.sla.0000189115.94847.f1
The Notch signaling pathway is related to neurovascular progression of pancreatic cancer
Abstract
Objective: To analyze the potential role of the Notch signaling pathway in pancreatic cancer angiogenesis and invasion.
Background: Angiogenesis, pain, and early neuroinvasion are clinical features of pancreatic cancer. Blood vessels and nerves develop together and use common routes through the organism. The Notch pathway (Notch-1/4, Jagged-1/2, Delta-1) appears crucial in this process. The current study analyzed the Notch pathway in pancreatic cancer and characterized its angiogenic and invasive effects.
Methods: Five PaCa cell lines were cultured for the in vitro experiments. Real-time quantitative RT-PCR was done to quantify mRNA expression in 31 human PaCa specimens, and immunohistochemistry was used to localize protein expression within tumor specimens. Activation of the Notch signaling was done by transfection of PaCa cells with a constitutive active Notch-1 mutant (Notch-IC). Overexpression of Jagged and Delta was achieved by transfection of full-length cDNA. Spheroid assays were used to study angiogenesis and ELISAs to measure VEGF, bFGF, and angiogenin expression. Matrigel invasion assays were used to analyze tumor cell invasion.
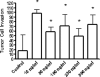
Results: Notch-3 and Notch-4 mRNA were significantly (P < 0.001) overexpressed in PaCa. Immunohistochemistry revealed protein accumulation of Notch-1 as well. All ligands were significantly up-regulated. A positive immunosignal of ligands was seen in nerves, blood vessels, and ductal tumor cells. Transfection of PaCa cells with the constitutive active Notch-IC mutant and with Jagged-1 revealed increased levels for VEGF. Concomitantly, recombinant Jagged-1 increased sprouting of endothelial cells in the spheroid assay.
Conclusion: The Notch pathway most likely regulates neurovascular development in pancreatic cancer. Activation of this signaling pathway by constitutive Notch-1 mutants and by Jagged-1 causes an angiogenic and invasive tumor phenotype. Specific blockade of Notch signaling may therefore be beneficial for patients with pancreatic cancer.
Figures

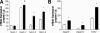




References
-
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. - PubMed
-
- Brat DJ, Lillemoe KD, Yeo CJ, et al. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–169. - PubMed
-
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

