In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells
- PMID: 16327496
- PMCID: PMC1409890
- DOI: 10.1097/01.sla.0000189572.02554.2c
In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells
Abstract
Objective: To compare the in vitro osteogenic differentiation and in vivo ectopic bone forming capacity of human bone marrow stromal cells (BMSCs) and jaw periosteal cells (JPCs), and to identify molecular predictors of their osteogenic capacity.
Summary background data: JPC could be an appealing alternative to BMSC for the engineering of cell-based osteoinductive grafts because of the relatively easy access to tissue with minimal morbidity. However, the extent of osteogenic capacity of JPC has not yet been established or compared with that of BMSC.
Methods: BMSCs and JPCs from the same donors (N = 9), expanded for 2 passages, were cultured for 3 weeks in osteogenic medium either in monolayers (Model I) or within 3-dimensional porous ceramic scaffolds, following embedding in fibrin gel (Model II). Cell-fibrin-ceramic constructs were also implanted ectopically in nude mice for 8 weeks (Model III). Cell differentiation in vitro was assessed biochemically and by real-time RT-PCR. Bone formation in vivo was quantified by computerized histomorphometry.
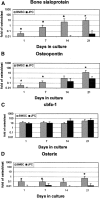
Results: JPCs had lower alkaline phosphatase activity, deposited smaller amounts of calcium (Model I), and expressed lower mRNA levels of bone sialoprotein, osteopontin, and osterix (Models I and II) than BMSCs. JPCs produced ectopic bone tissue at lower frequency and amounts (Model III) than BMSCs. Bone sialoprotein, osteopontin, and osterix mRNA levels by BMSCs or JPCs in Model II were markedly higher than in Model I and significantly more expressed by cells that generated bone tissue in Model III.
Conclusions: Our data indicate that JPCs, although displaying features of osteogenic cells, would not be as reliable as BMSCs for cell-based bone tissue engineering, and suggest that expression of osteoblast-related markers in vitro could be used to predict whether cells would be osteoinductive in vivo.
Figures

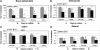



References
-
- Schnurer SM, Gopp U, Kuhn KD, et al. Bone substitutes. Orthopade. 2003;32:2–10. - PubMed
-
- Jaquiery C, Rohner D, Kunz C, et al. Reconstruction of maxillary and mandibular defects using prefabricated microvascular fibular grafts and osseointegrated dental implants: a prospective study. Clin Oral Implants Res. 2004;15:598–606. - PubMed
-
- Hartman EH, Spauwen PH, Jansen JA. Donor-site complications in vascularized bone flap surgery. J Invest Surg. 2002;15:185–197. - PubMed
-
- Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

