Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development
- PMID: 16330359
- PMCID: PMC1315066
- DOI: 10.1289/ehp.8230
Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development
Abstract
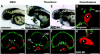
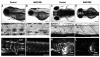
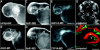
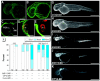
Polycyclic aromatic hydrocarbons (PAHs), derived largely from fossil fuels and their combustion, are pervasive contaminants in rivers, lakes, and nearshore marine habitats. Studies after the Exxon Valdez oil spill demonstrated that fish embryos exposed to low levels of PAHs in weathered crude oil develop a syndrome of edema and craniofacial and body axis defects. Although mechanisms leading to these defects are poorly understood, it is widely held that PAH toxicity is linked to aryl hydrocarbon receptor (AhR) binding and cytochrome P450 1A (CYP1A) induction. Using zebrafish embryos, we show that the weathered crude oil syndrome is distinct from the well-characterized AhR-dependent effects of dioxin toxicity. Blockade of AhR pathway components with antisense morpholino oligonucleotides demonstrated that the key developmental defects induced by weathered crude oil exposure are mediated by low-molecular-weight tricyclic PAHs through AhR-independent disruption of cardiovascular function and morphogenesis. These findings have multiple implications for the assessment of PAH impacts on coastal habitats.
Figures

References
-
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol Pharmacol. 2002;62:234–249. - PubMed
-
- Barron MG, Heintz RA, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. - PubMed
-
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev Dyn. 2001;222:581–594. - PubMed
-
- Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

