Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans
- PMID: 16330766
- PMCID: PMC1306794
- DOI: 10.1073/pnas.0506624102
Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans
Abstract
Abnormal processing of somatosensory inputs in the central nervous system (central sensitization) is the mechanism accounting for the enhanced pain sensitivity in the skin surrounding tissue injury (secondary hyperalgesia). Secondary hyperalgesia shares clinical characteristics with neurogenic hyperalgesia in patients with neuropathic pain. Abnormal brain responses to somatosensory stimuli have been found in patients with hyperalgesia as well as in normal subjects during experimental central sensitization. The aim of this study was to assess the effects of gabapentin, a drug effective in neuropathic pain patients, on brain processing of nociceptive information in normal and central sensitization states. Using functional magnetic resonance imaging (fMRI) in normal volunteers, we studied the gabapentin-induced modulation of brain activity in response to nociceptive mechanical stimulation of normal skin and capsaicin-induced secondary hyperalgesia. The dose of gabapentin was 1,800 mg per os, in a single administration. We found that (i) gabapentin reduced the activations in the bilateral operculoinsular cortex, independently of the presence of central sensitization; (ii) gabapentin reduced the activation in the brainstem, only during central sensitization; (iii) gabapentin suppressed stimulus-induced deactivations, only during central sensitization; this effect was more robust than the effect on brain activation. The observed drug-induced effects were not due to changes in the baseline fMRI signal. These findings indicate that gabapentin has a measurable antinociceptive effect and a stronger antihyperalgesic effect most evident in the brain areas undergoing deactivation, thus supporting the concept that gabapentin is more effective in modulating nociceptive transmission when central sensitization is present.
Figures
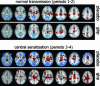

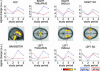
Comment in
-
Pains, gains, and midbrains.Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17885-6. doi: 10.1073/pnas.0508494102. Epub 2005 Dec 5. Proc Natl Acad Sci U S A. 2005. PMID: 16330782 Free PMC article. No abstract available.
References
-
- Lewis, T. (1936) Clin. Sci. 2, 373-421.
-
- Meyer, R. A. & Treede, R. D. (2004) in Hyperalgesia: Molecular Mechanisms and Clinical Implications, eds. Brune, K. & Handwerker, H. O. (IASP Press, Seattle), Vol. 30, pp. 143-155.
-
- Magerl, W., Fuchs, P. N., Meyer, R. A. & Treede, R. D. (2001) Brain 124, 1754-1764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

