Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair
- PMID: 16330768
- PMCID: PMC1312388
- DOI: 10.1073/pnas.0506535102
Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair
Abstract
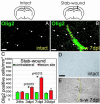
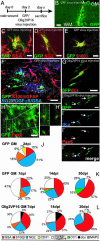
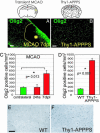
Despite the presence of neural stem cells and ongoing neurogenesis in some regions of the adult mammalian brain, neurons are not replaced in most brain regions after injury. With the aim to unravel factors contributing to the failure of neurogenesis in the injured cerebral cortex, we examined the expression of cell fate determinants after acute brain injuries, such as stab wound or focal ischemia, and in a model of chronic amyloid deposition. Although none of the neurogenic factors, such as Pax6, Mash1, Ngn2, was detected in the injured parenchyma, we observed a strong up-regulation of the bHLH transcription factor Olig2, but not Olig1, upon acute and chronic injury. To examine the function of Olig2 in brain lesion, we injected retroviral vectors containing a dominant negative form of Olig2 into the lesioned cortex 2 days after a stab wound. Antagonizing Olig2 function resulted in a significant number of infected cells generating immature neurons that were not observed after injection of the control virus. These data, therefore, imply Olig2 as a repressor of neurogenesis in cells reacting to brain injury and open innovative perspectives toward evoking endogenous neuronal repair.
Figures

References
-
- Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Cell 97, 703-716. - PubMed
-
- Garcia, A. D., Doan, N. B., Imura, T., Bush, T. G. & Sofroniew, M. V. (2004) Nat. Neurosci. 7, 1233-1241. - PubMed
-
- Fawcett, J. W. & Asher, R. A. (1999) Brain Res. Bull. 49, 377-391. - PubMed
-
- Ridet, J. L., Malhotra, S. K., Privat, A. & Gage, F. H. (1997) Trends Neurosci. 20, 570-577. - PubMed
-
- Hartfuss, E., Galli, R., Heins, N. & Götz, M. (2001) Dev. Biol. 229, 15-30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

