Investigation of the binding geometry of a peripheral membrane protein
- PMID: 16331966
- PMCID: PMC2516348
- DOI: 10.1021/bi051127+
Investigation of the binding geometry of a peripheral membrane protein
Abstract
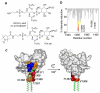
A growing number of modules including FYVE domains target key signaling proteins to membranes through specific recognition of lipid headgroups and hydrophobic insertion into bilayers. Despite the critical role of membrane insertion in the function of these modules, the structural mechanism of membrane docking and penetration remains unclear. In particular, the three-dimensional orientation of the inserted proteins with respect to the membrane surface is difficult to define quantitatively. Here, we determined the geometry of the micelle penetration of the early endosome antigen 1 (EEA1) FYVE domain by obtaining NMR-derived restraints that correlate with the distances between protein backbone amides and spin-labeled probes. The 5- and 14-doxyl-phosphatidylcholine spin-labels were incorporated into dodecylphosphocholine (DPC) micelles, and the reduction of amide signal intensities of the FYVE domain due to paramagnetic relaxation enhancement was measured. The vector of the FYVE domain insertion was estimated relative to the molecular axis by minimizing the paramagnetic restraints obtained in phosphatidylinositol 3-phosphate (PI3P)-enriched micelles containing only DPC or mixed with phosphatidylserine (PS). Additional distance restraints were obtained using a novel spin-label mimetic of PI(3)P that contains a nitroxyl radical near the threitol group of the lipid. Conformational changes indicative of elongation of the membrane insertion loop (MIL) were detected upon micelle interaction, in which the hydrophobic residues of the loop tend to move deeper into the nonpolar core of micelles. The micelle insertion mechanism of the FYVE domain defined in this study is consistent with mutagenesis data and chemical shift perturbations and demonstrates the advantage of using the spin-label NMR approach for investigating the binding geometry by peripheral membrane proteins.
Figures




Similar articles
-
Phosphatidylinositol 3-phosphate recognition and membrane docking by the FYVE domain.Biochim Biophys Acta. 2006 Aug;1761(8):868-77. doi: 10.1016/j.bbalip.2006.03.011. Epub 2006 Apr 7. Biochim Biophys Acta. 2006. PMID: 16644267 Free PMC article. Review.
-
Multivalent mechanism of membrane insertion by the FYVE domain.J Biol Chem. 2004 Jan 23;279(4):3050-7. doi: 10.1074/jbc.M309007200. Epub 2003 Oct 25. J Biol Chem. 2004. PMID: 14578346
-
Structural mechanism of endosome docking by the FYVE domain.Science. 2001 Mar 2;291(5509):1793-6. doi: 10.1126/science.291.5509.1793. Science. 2001. PMID: 11230696
-
Lipid interaction networks of peripheral membrane proteins revealed by data-driven micelle docking.Biophys J. 2008 Jan 15;94(2):515-24. doi: 10.1529/biophysj.107.115923. Epub 2007 Sep 21. Biophys J. 2008. PMID: 17890395 Free PMC article.
-
Peripheral membrane proteins: FYVE sticky fingers.Curr Biol. 1999 Nov 18;9(22):R857-60. doi: 10.1016/s0960-9822(00)80046-9. Curr Biol. 1999. PMID: 10574756 Review.
Cited by
-
Mechanistic similarities in docking of the FYVE and PX domains to phosphatidylinositol 3-phosphate containing membranes.Prog Lipid Res. 2007 Nov;46(6):315-27. doi: 10.1016/j.plipres.2007.06.001. Epub 2007 Jul 13. Prog Lipid Res. 2007. PMID: 17707914 Free PMC article. Review.
-
Membrane binding domains.Biochim Biophys Acta. 2006 Aug;1761(8):805-11. doi: 10.1016/j.bbalip.2006.02.020. Epub 2006 Mar 24. Biochim Biophys Acta. 2006. PMID: 16616874 Free PMC article. Review.
-
Phosphatidylinositol 3-phosphate recognition and membrane docking by the FYVE domain.Biochim Biophys Acta. 2006 Aug;1761(8):868-77. doi: 10.1016/j.bbalip.2006.03.011. Epub 2006 Apr 7. Biochim Biophys Acta. 2006. PMID: 16644267 Free PMC article. Review.
-
Stabilized phosphatidylinositol-5-phosphate analogues as ligands for the nuclear protein ING2: chemistry, biology, and molecular modeling.J Am Chem Soc. 2007 May 23;129(20):6498-506. doi: 10.1021/ja070195b. Epub 2007 May 1. J Am Chem Soc. 2007. PMID: 17469822 Free PMC article.
-
The role of hydrophobic interactions in positioning of peripheral proteins in membranes.BMC Struct Biol. 2007 Jun 29;7:44. doi: 10.1186/1472-6807-7-44. BMC Struct Biol. 2007. PMID: 17603894 Free PMC article.
References
-
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–13. - PubMed
-
- Overduin M, Cheever ML, Kutateladze TG. Signaling with phosphoinositides: better than binary. Molecular interventions. 2001;1:150–9. - PubMed
-
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin.[comment] Nature. 2002;419:361–6. - PubMed
-
- Stahelin RV, Long F, Peter BJ, Murray D, De Camilli P, McMahon HT, Cho W. Contrasting membrane interaction mechanisma of AP180 N-terminal homology (ANTH) and Epsin N-terminal homology (ENTH) domains. Journal of Biological Chemistry. 2003;278:28993–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

