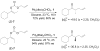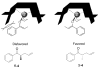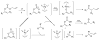Palladium-catalyzed asymmetric allylic alpha-alkylation of acyclic ketones
- PMID: 16332054
- PMCID: PMC2533158
- DOI: 10.1021/ja055968f
Palladium-catalyzed asymmetric allylic alpha-alkylation of acyclic ketones
Abstract
The first example of Pd-catalyzed asymmetric allyl alkylation of the conformationally nonrigid acyclic ketone enolates is reported with excellent yields, regioselectivity, and enantioselectivity. The double bond geometry of the allyl enol carbonates affects its reactivity, selectivity, as well as the absolute configuration of the products. An opposite enantioselectivity from what is predicted by a direct attack of the enolate on the allyl moiety of the pi-ally-Pd complex was observed. An alternative mechanism was proposed, which involves an inner sphere process of coordination of the enolate to Pd followed by reductive elimination.
Figures
References
-
- Caine D. In: Comprehensive Organic Synthesis: Carbon-Carbon σ-bond formation. Trost BM, Fleming I, editors. Vol. 3 Pergamon; New York: 1991.
-
- House HO. Modern Synthetic Reactions. 2. Benjamin, W. A; Menlo Park, CA: 1972. pp. 492–628.
-
-
Polyalkylation was found in other substrates. See Tsuji J, Yamada T, Minami I, Nisar M, Shimizu IJ. Org Chem. 1987;52:29–88.Shimizu I, Minami I, Tsuji J. Tetrahedron Lett. 1983;24:1797.
-
-
- Masamune S, Ellingboe JW, Choy W. J Am Chem Soc. 1982;104:5526.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous