A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding
- PMID: 16332249
- PMCID: PMC1383718
- DOI: 10.1042/BJ20051563
A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding
Abstract
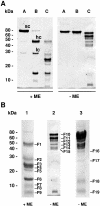
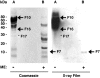
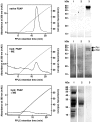
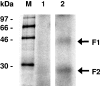
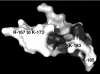
FSAP (Factor VII-activating protease) is a novel plasma-derived serine protease that regulates haemostasis as well as vascular cell proliferation. FSAP undergoes autoactivation in the presence of polyanionic macromolecules such as heparin and RNA. Competition experiments suggest that RNA and heparin bind to the same or overlapping interaction sites. A proteolysis approach, where FSAP was hydrolysed into smaller fragments, was used to identify the polyanion-binding site. The EGF (epidermal growth factor)-like domains EGF2 and EGF3 of FSAP are the major interaction domains for RNA. The amino acids Arg170, Arg171, Ser172 and Lys173 within the EGF3 domain were essential for this binding. This is also the region with the highest positive net charge in the protein and is most probably located in an exposed loop. It is also highly conserved across five species. Disruption of disulphide bridges led to the loss of RNA and heparin binding, indicating that the three-dimensional structure of the EGF3 domain is essential for binding to negatively charged heparin or RNA. The identification of polyanion-binding sites will help to define the role of FSAP in the vasculature.
Figures


Similar articles
-
Extracellular RNA is a natural cofactor for the (auto-)activation of Factor VII-activating protease (FSAP).Biochem J. 2005 Feb 1;385(Pt 3):831-8. doi: 10.1042/BJ20041021. Biochem J. 2005. PMID: 15654766 Free PMC article.
-
High negative charge-to-size ratio in polyphosphates and heparin regulates factor VII-activating protease.FEBS J. 2009 Sep;276(17):4828-39. doi: 10.1111/j.1742-4658.2009.07183.x. Epub 2009 Jul 31. FEBS J. 2009. PMID: 19664058
-
Nucleic acids potentiate Factor VII-activating protease (FSAP)-mediated cleavage of platelet-derived growth factor-BB and inhibition of vascular smooth muscle cell proliferation.Biochem J. 2007 May 15;404(1):45-50. doi: 10.1042/BJ20070166. Biochem J. 2007. PMID: 17300216 Free PMC article.
-
Factor VII activating protease (FSAP): a novel protective factor in liver fibrosis.Proteomics Clin Appl. 2014 Jun;8(5-6):438-46. doi: 10.1002/prca.201300078. Epub 2014 Mar 26. Proteomics Clin Appl. 2014. PMID: 24497464 Review.
-
Factor VII activating protease (FSAP): a novel protease in hemostasis.Biol Chem. 2002 Jul-Aug;383(7-8):1119-24. doi: 10.1515/BC.2002.121. Biol Chem. 2002. PMID: 12437095 Review.
Cited by
-
Multifunctional Cationic Hyperbranched Polyaminoglycosides that Target Multiple Mediators for Severe Abdominal Trauma Management.Adv Sci (Weinh). 2024 Jan;11(1):e2305273. doi: 10.1002/advs.202305273. Epub 2023 Nov 23. Adv Sci (Weinh). 2024. PMID: 37997512 Free PMC article.
-
Inhibition of PDGF-BB by Factor VII-activating protease (FSAP) is neutralized by protease nexin-1, and the FSAP-inhibitor complexes are internalized via LRP.Biochem J. 2007 Jun 1;404(2):191-6. doi: 10.1042/BJ20061630. Biochem J. 2007. PMID: 17298300 Free PMC article.
-
Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis-Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review.Int J Mol Sci. 2023 Mar 13;24(6):5473. doi: 10.3390/ijms24065473. Int J Mol Sci. 2023. PMID: 36982544 Free PMC article. Review.
-
Targeted Therapy with Cisplatin-Loaded Calcium Citrate Nanoparticles Conjugated with Epidermal Growth Factor for Lung Cancer Treatment.ACS Omega. 2024 Jun 9;9(24):25668-25677. doi: 10.1021/acsomega.3c08969. eCollection 2024 Jun 18. ACS Omega. 2024. PMID: 38911765 Free PMC article.
-
Hyaluronic Acid binding protein 2 is a novel regulator of vascular integrity.Arterioscler Thromb Vasc Biol. 2010 Mar;30(3):483-90. doi: 10.1161/ATVBAHA.109.200451. Epub 2009 Dec 30. Arterioscler Thromb Vasc Biol. 2010. PMID: 20042707 Free PMC article.
References
-
- Kannemeier C., Feussner A., Stöhr H. A., Weisse J., Preissner K. T., Römisch J. Factor VII and single-chain plasminogen activator-activating protease: activation and autoactivation of the proenzyme. Eur. J. Biochem. 2001;268:3789–3796. - PubMed
-
- Römisch J. Factor VII activating protease (FSAP): a novel protease in hemostasis. Biol. Chem. 2002;383:1119–1124. - PubMed
-
- Choi-Miura N. H., Tobe T., Sumiya J. I., Nakano Y., Sano Y., Mazda T., Tomita M. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: it has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J. Biochem. (Tokyo) 1996;119:1157–1165. - PubMed
-
- Sumiya J. I., Asakawa S., Tobe T., Hashimoto K., Saguchi K. I., Choi-Miura N. H., Shimizu Y., Minoshima S., Shimizu N., Tomita M. Isolation and characterization of the plasma hyaluronan-binding protein (PHBP) gene (HABP2) J. Biochem. (Tokyo) 1997;122:983–990. - PubMed
-
- Etscheid M., Hunfeld A., Konig H., Seitz R., Dodt J. Activation of proPHBSP, the zymogen of a plasma hyaluronan binding serine protease, by an intermolecular autocatalytic mechanism. Biol. Chem. 2000;381:1223–1231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

